GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
fibroblast growth factor receptor 3
Target id: 1810
Nomenclature: fibroblast growth factor receptor 3
Abbreviated Name: FGFR3
Family: Type V RTKs: FGF (fibroblast growth factor) receptor family
Contents:
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 1 | 806 | 4p16.3 | FGFR3 | fibroblast growth factor receptor 3 | |
| Mouse | 1 | 801 | 5 17.83 cM | Fgfr3 | fibroblast growth factor receptor 3 | |
| Rat | - | 800 | 14q21 | Fgfr3 | fibroblast growth factor receptor 3 | |
Previous and Unofficial Names  |
| achondroplasia, thanatophoric dwarfism | HBGFR | sam3 | Heparin-binding growth factor receptor | ACH | CD333 | CEK2 | JTK4 |
Database Links  |
|
| Alphafold | P22607 (Hs), Q61851 (Mm) |
| BRENDA | 2.7.10.1 |
| CATH/Gene3D | 2.60.40.10 |
| ChEMBL Target | CHEMBL2742 (Hs), CHEMBL4066 (Mm) |
| Ensembl Gene | ENSG00000068078 (Hs), ENSMUSG00000054252 (Mm), ENSRNOG00000016818 (Rn) |
| Entrez Gene | 2261 (Hs), 14184 (Mm), 84489 (Rn) |
| Human Protein Atlas | ENSG00000068078 (Hs) |
| KEGG Enzyme | 2.7.10.1 |
| KEGG Gene | hsa:2261 (Hs), mmu:14184 (Mm), rno:84489 (Rn) |
| OMIM | 134934 (Hs) |
| Orphanet | ORPHA121815 (Hs) |
| Pharos | P22607 (Hs) |
| RefSeq Nucleotide | NM_000142 (Hs), NM_001205270 (Mm), NM_053429 (Rn) |
| RefSeq Protein | NP_000133 (Hs), NP_001156688 (Mm), NP_001156689 (Mm), NP_001192199 (Mm), NP_001156687 (Mm), NP_032036 (Mm), NP_445881 (Rn) |
| UniProtKB | P22607 (Hs), Q61851 (Mm) |
| Wikipedia | FGFR3 (Hs) |
Selected 3D Structures  |
|||||||||||
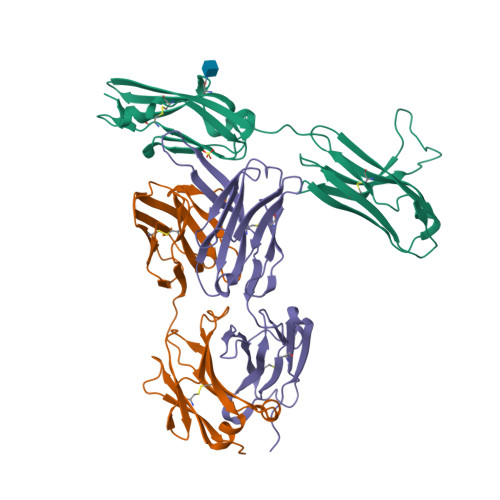
|
|
||||||||||
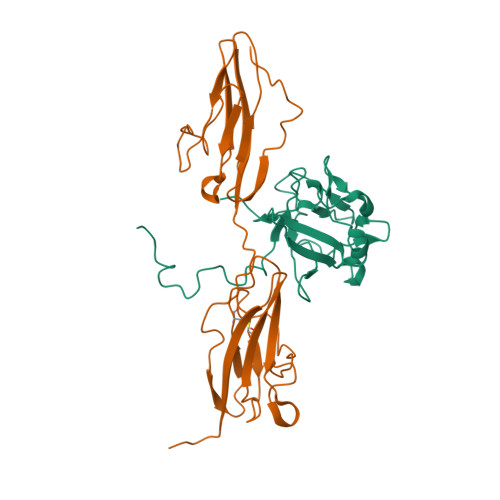
|
|
||||||||||
Enzyme Reaction  |
||||
|
||||
| Endogenous ligand (Human) |
| FGF-3 (FGF3, P11487) |
| Endogenous ligands (Human) |
| FGF-1 (FGF1, P05230), FGF-2 (FGF2, P09038), FGF-9 (FGF9, P31371) > FGF-4 (FGF4, P08620), FGF-8 (FGF8, P55075) [21] |
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DiscoveRx KINOMEscan® screen  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen of 72 inhibitors against 456 human kinases. Quantitative data were derived using DiscoveRx KINOMEscan® platform. http://www.discoverx.com/services/drug-discovery-development-services/kinase-profiling/kinomescan Reference: 6,32 |
 
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: FGFR3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: FGFR3(G697C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EMD Millipore KinaseProfilerTM screen/Reaction Biology Kinase HotspotSM screen  |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen profiling 158 kinase inhibitors (Calbiochem Protein Kinase Inhibitor Library I and II, catalogue numbers 539744 and 539745) for their inhibitory activity at 1µM and 10µM against 234 human recombinant kinases using the EMD Millipore KinaseProfilerTM service. A screen profiling the inhibitory activity of 178 commercially available kinase inhibitors at 0.5µM against a panel of 300 recombinant protein kinases using the Reaction Biology Corporation Kinase HotspotSM platform. http://www.millipore.com/techpublications/tech1/pf3036 http://www.reactionbiology.com/webapps/main/pages/kinase.aspx Reference: 1,8 |
 
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: FGFR3/FGFR3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
References
1. Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. (2011) Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1039-45. [PMID:22037377]
2. AstraZeneca. AZD4547. Accessed on 11/09/2014. Modified on 11/09/2014. astrazeneca.com, http://openinnovation.astrazeneca.com/what-we-offer/compound/azd4547/
3. Brameld KA, Owens TD, Verner E, Venetsanakos E, Bradshaw JM, Phan VT, Tam D, Leung K, Shu J, LaStant J et al.. (2017) Discovery of the Irreversible Covalent FGFR Inhibitor 8-(3-(4-Acryloylpiperazin-1-yl)propyl)-6-(2,6-dichloro-3,5-dimethoxyphenyl)-2-(methylamino)pyrido[2,3-d]pyrimidin-7(8H)-one (PRN1371) for the Treatment of Solid Tumors. J Med Chem, 60 (15): 6516-6527. [PMID:28665128]
4. Brohm D, Heroult M, Collin M-P, Hübsch W, Lobell M, Lustig K, Grünewald S, Bömer U, Voehringer V. (2013) Disubstituted benzothienyl-pyrrolotriazines and their use as FGFR kinase inhibitors. Patent number: WO2013087578. Assignee: Bayer Intellectual Property Gmbh. Priority date: 15/12/2011. Publication date: 20/06/2013.
5. Chesi M, Nardini E, Brents LA, Schröck E, Ried T, Kuehl WM, Bergsagel PL. (1997) Frequent translocation t(4;14)(p16.3;q32.3) in multiple myeloma is associated with increased expression and activating mutations of fibroblast growth factor receptor 3. Nat Genet, 16 (3): 260-4. [PMID:9207791]
6. Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. (2011) Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1046-51. [PMID:22037378]
7. Funasaka S, Okada S, Tanaka K, Nagao S, Ohashi I, Yamane Y, Nakatani Y,Karouji Y. (2014) Monocyclic pyridine derivative. Patent number: WO2014129477A1. Assignee: Eisai R & D. Priority date: 20/02/2013. Publication date: 28/08/2014.
8. Gao Y, Davies SP, Augustin M, Woodward A, Patel UA, Kovelman R, Harvey KJ. (2013) A broad activity screen in support of a chemogenomic map for kinase signalling research and drug discovery. Biochem J, 451 (2): 313-28. [PMID:23398362]
9. Goriely A, Hansen RM, Taylor IB, Olesen IA, Jacobsen GK, McGowan SJ, Pfeifer SP, McVean GA, Rajpert-De Meyts E, Wilkie AO. (2009) Activating mutations in FGFR3 and HRAS reveal a shared genetic origin for congenital disorders and testicular tumors. Nat Genet, 41 (11): 1247-52. [PMID:19855393]
10. Guagnano V, Furet P, Spanka C, Bordas V, Le Douget M, Stamm C, Brueggen J, Jensen MR, Schnell C, Schmid H et al.. (2011) Discovery of 3-(2,6-dichloro-3,5-dimethoxy-phenyl)-1-{6-[4-(4-ethyl-piperazin-1-yl)-phenylamino]-pyrimidin-4-yl}-1-methyl-urea (NVP-BGJ398), a potent and selective inhibitor of the fibroblast growth factor receptor family of receptor tyrosine kinase. J Med Chem, 54 (20): 7066-83. [PMID:21936542]
11. Hagel M, Miduturu C, Sheets M, Rubin N, Weng W, Stransky N, Bifulco N, Kim JL, Hodous B, Brooijmans N et al.. (2015) First Selective Small Molecule Inhibitor of FGFR4 for the Treatment of Hepatocellular Carcinomas with an Activated FGFR4 Signaling Pathway. Cancer Discov, 5 (4): 424-37. [PMID:25776529]
12. Hall TG, Yu Y, Eathiraj S, Wang Y, Savage RE, Lapierre JM, Schwartz B, Abbadessa G. (2016) Preclinical Activity of ARQ 087, a Novel Inhibitor Targeting FGFR Dysregulation. PLoS ONE, 11 (9): e0162594. [PMID:27627808]
13. Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U, Garin-Chesa P, Bader G, Zoephel A, Quant J et al.. (2008) BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res, 68 (12): 4774-82. [PMID:18559524]
14. Hudkins RL, Allen E, Balcer A, Hoffman ID, Iyer S, Neal M, Nelson KJ, Rideout M, Ye Q, Starrett JH et al.. (2024) Discovery of TYRA-300: First Oral Selective FGFR3 Inhibitor for the Treatment of Urothelial Cancers and Achondroplasia. J Med Chem, 67 (18): 16737-16756. [PMID:39258897]
15. Hudkins RL, Bensen DC. (2022) Aminopyrimidine compounds and methods of their use. Patent number: WO2022182972A1. Assignee: Tyra Biosciences, Inc.. Priority date: 25/02/2022. Publication date: 01/09/2022.
16. Kuriwaki I, Kameda M, Iikubo K, Hisamichi H, Kawamoto Y, Kikuchi S, Moritomo H, Terasaka T, Iwai Y, Noda A et al.. (2022) Discovery of ASP5878: Synthesis and structure-activity relationships of pyrimidine derivatives as pan-FGFRs inhibitors with improved metabolic stability and suppressed hERG channel inhibitory activity. Bioorg Med Chem, 59: 116657. [PMID:35219181]
17. Nakanishi Y, Akiyama N, Tsukaguchi T, Fujii T, Sakata K, Sase H, Isobe T, Morikami K, Shindoh H, Mio T et al.. (2014) The fibroblast growth factor receptor genetic status as a potential predictor of the sensitivity to CH5183284/Debio 1347, a novel selective FGFR inhibitor. Mol Cancer Ther, 13 (11): 2547-58. [PMID:25169980]
18. Nguyen MH, Ye HF, Xu Y, Truong L, Horsey A, Styduhar ED, Frascella M, Leffet L, Federowicz K, Behshad E et al.. (2023) Discovery of Orally Bioavailable FGFR2/FGFR3 Dual Inhibitors via Structure-Guided Scaffold Repurposing Approach. ACS Medicinal Chemistry Letters, 14 (3): 312–318. DOI: 10.1021/acsmedchemlett.3c00003
19. Okaniwa M, Hirose M, Arita T, Yabuki M, Nakamura A, Takagi T, Kawamoto T, Uchiyama N, Sumita A, Tsutsumi S et al.. (2013) Discovery of a selective kinase inhibitor (TAK-632) targeting pan-RAF inhibition: design, synthesis, and biological evaluation of C-7-substituted 1,3-benzothiazole derivatives. J Med Chem, 56 (16): 6478-94. [PMID:23906342]
20. Olsen SK, Ibrahimi OA, Raucci A, Zhang F, Eliseenkova AV, Yayon A, Basilico C, Linhardt RJ, Schlessinger J, Mohammadi M. (2004) Insights into the molecular basis for fibroblast growth factor receptor autoinhibition and ligand-binding promiscuity. Proc Natl Acad Sci USA, 101 (4): 935-40. [PMID:14732692]
21. Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M. (1996) Receptor specificity of the fibroblast growth factor family. J Biol Chem, 271 (25): 15292-7. [PMID:8663044]
22. Pan BS, Chan GK, Chenard M, Chi A, Davis LJ, Deshmukh SV, Gibbs JB, Gil S, Hang G, Hatch H et al.. (2010) MK-2461, a novel multitargeted kinase inhibitor, preferentially inhibits the activated c-Met receptor. Cancer Res, 70 (4): 1524-33. [PMID:20145145]
23. Peng X, Hou P, Chen Y, Dai Y, Ji Y, Shen Y, Su Y, Liu B, Wang Y, Sun D et al.. (2019) Preclinical evaluation of 3D185, a novel potent inhibitor of FGFR1/2/3 and CSF-1R, in FGFR-dependent and macrophage-dominant cancer models. J Exp Clin Cancer Res, 38 (1): 372. [PMID:31438996]
24. Qing J, Du X, Chen Y, Chan P, Li H, Wu P, Marsters S, Stawicki S, Tien J, Totpal K et al.. (2009) Antibody-based targeting of FGFR3 in bladder carcinoma and t(4;14)-positive multiple myeloma in mice. J Clin Invest, 119 (5): 1216-29. [PMID:19381019]
25. Renhowe PA, Pecchi S, Shafer CM, Machajewski TD, Jazan EM, Taylor C, Antonios-McCrea W, McBride CM, Frazier K, Wiesmann M et al.. (2009) Design, structure-activity relationships and in vivo characterization of 4-amino-3-benzimidazol-2-ylhydroquinolin-2-ones: a novel class of receptor tyrosine kinase inhibitors. J Med Chem, 52 (2): 278-92. [PMID:19113866]
26. Sagara T, Ito S, Otsuki S, Sootome H. (2013) 3,5-disubstituted alkynylbenzene compound and salt thereof. Patent number: WO2013108809. Assignee: Taiho Pharmaceutical Co., Ltd.. Priority date: 19/01/2012. Publication date: 25/07/2013.
27. Saxty G, Murray CW, Berdini V, Besong GE, Hamlett CCF, Johnson CN, Woodhead SJ, Reader M, Rees DC, Mevellec LA et al.. (2011) Pyrazolyl quinazoline kinase inhibitors. Patent number: WO2011135376 A1. Assignee: Astex Therapeutics Limited. Priority date: 30/04/2010. Publication date: 03/11/2011.
28. Shvartsbart A, Roach JJ, Witten MR, Koblish H, Harris JJ, Covington M, Hess R, Lin L, Frascella M, Truong L et al.. (2022) Discovery of Potent and Selective Inhibitors of Wild-Type and Gatekeeper Mutant Fibroblast Growth Factor Receptor (FGFR) 2/3. J Med Chem, 65 (22): 15433-15442. [PMID:36356320]
29. Su WG, Zhang W, Li J. (2014) Novel pyrimidine and pyridine compounds and their usage. Patent number: WO2014139465A1. Assignee: Hutchison Medipharma Limited. Priority date: 14/03/2014. Publication date: 18/09/2014.
30. Trudel S, Li ZH, Wei E, Wiesmann M, Chang H, Chen C, Reece D, Heise C, Stewart AK. (2005) CHIR-258, a novel, multitargeted tyrosine kinase inhibitor for the potential treatment of t(4;14) multiple myeloma. Blood, 105 (7): 2941-8. [PMID:15598814]
31. Wang T, Ioannidis S, Almeida L, Block MH, Davies AM, Lamb ML, Scott DA, Su M, Zhang HJ, Alimzhanov M et al.. (2011) In vitro and in vivo evaluation of 6-aminopyrazolyl-pyridine-3-carbonitriles as JAK2 kinase inhibitors. Bioorg Med Chem Lett, 21 (10): 2958-61. [PMID:21493067]
32. Wodicka LM, Ciceri P, Davis MI, Hunt JP, Floyd M, Salerno S, Hua XH, Ford JM, Armstrong RC, Zarrinkar PP et al.. (2010) Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. Chem Biol, 17 (11): 1241-9. [PMID:21095574]
33. Wu F. (2021) Multi-kinase inhibitor compound, and crystal form and use thereof. Patent number: US10889586B2. Assignee: Nanjing Transthera Biosciences Co Ltd. Priority date: 13/12/2016. Publication date: 12/01/2021.
34. Wu L, Zhang C, He C, Sun Y, Lu L, Qian D-Q, Xu M, Zhou J, Yao W. (2014) Substituted tricyclic compounds as FGFR inhibitors. Patent number: WO2014007951. Assignee: Incyte Corporation. Priority date: 13/06/2012. Publication date: 09/01/2014.
35. Zhao G, Li WY, Chen D, Henry JR, Li HY, Chen Z, Zia-Ebrahimi M, Bloem L, Zhai Y, Huss K et al.. (2011) A novel, selective inhibitor of fibroblast growth factor receptors that shows a potent broad spectrum of antitumor activity in several tumor xenograft models. Mol Cancer Ther, 10 (11): 2200-10. [PMID:21900693]
How to cite this page
Type V RTKs: FGF (fibroblast growth factor) receptor family: fibroblast growth factor receptor 3. Last modified on 10/09/2025. Accessed on 31/01/2026. IUPHAR/BPS Guide to PHARMACOLOGY, https://www.guidetomalariapharmacology.org/GRAC/ObjectDisplayForward?objectId=1810.











