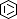GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
neurotrophic receptor tyrosine kinase 1
Target id: 1817
Nomenclature: neurotrophic receptor tyrosine kinase 1
Abbreviated Name: TrkA
Contents:
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 1 | 796 | 1q23.1 | NTRK1 | neurotrophic receptor tyrosine kinase 1 | |
| Mouse | 1 | 799 | 3 38.62 cM | Ntrk1 | neurotrophic tyrosine kinase, receptor, type 1 | |
| Rat | 1 | 799 | 2q34 | Ntrk1 | neurotrophic receptor tyrosine kinase 1 | |
Database Links  |
|
| Alphafold | P04629 (Hs), Q3UFB7 (Mm), P35739 (Rn) |
| BRENDA | 2.7.10.1 |
| CATH/Gene3D | 3.80.10.10, 2.60.40.10 |
| ChEMBL Target | CHEMBL2815 (Hs), CHEMBL4220 (Rn) |
| DrugBank Target | P04629 (Hs) |
| Ensembl Gene | ENSG00000198400 (Hs), ENSMUSG00000028072 (Mm), ENSRNOG00000013953 (Rn) |
| Entrez Gene | 4914 (Hs), 18211 (Mm), 59109 (Rn) |
| Human Protein Atlas | ENSG00000198400 (Hs) |
| KEGG Enzyme | 2.7.10.1 |
| KEGG Gene | hsa:4914 (Hs), mmu:18211 (Mm), rno:59109 (Rn) |
| OMIM | 191315 (Hs) |
| Orphanet | ORPHA123961 (Hs) |
| Pharos | P04629 (Hs) |
| RefSeq Nucleotide | NM_001007792 (Hs), NM_001033124 (Mm), NM_021589 (Rn) |
| RefSeq Protein | NP_001007793 (Hs), NP_001028296 (Mm), NP_067600 (Rn) |
| UniProtKB | P04629 (Hs), Q3UFB7 (Mm), P35739 (Rn) |
| Wikipedia | NTRK1 (Hs) |
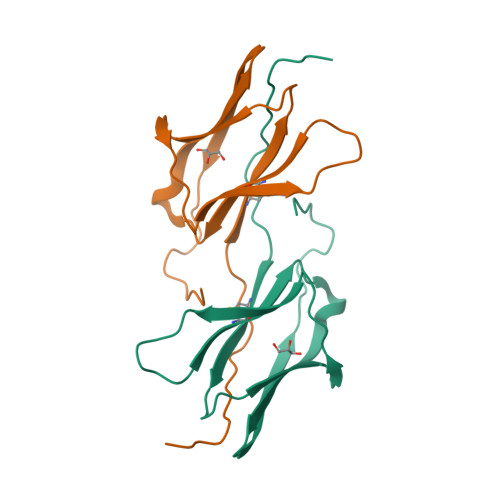
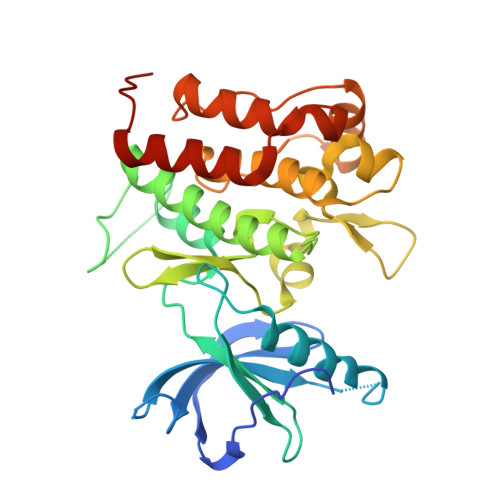
Selected 3D Structures  |
|||||||||||
|
|
||||||||||
|
|
||||||||||
|
|
||||||||||
Enzyme Reaction  |
||||
|
||||
| Endogenous ligands (Human) |
| NGF (NGF, P01138) > neurotrophin-3 (NTF3, P20783) |
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific inhibitor tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allosteric Modulators | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DiscoveRx KINOMEscan® screen  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen of 72 inhibitors against 456 human kinases. Quantitative data were derived using DiscoveRx KINOMEscan® platform. http://www.discoverx.com/services/drug-discovery-development-services/kinase-profiling/kinomescan Reference: 10,37 |
 
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: TRKA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EMD Millipore KinaseProfilerTM screen/Reaction Biology Kinase HotspotSM screen  |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen profiling 158 kinase inhibitors (Calbiochem Protein Kinase Inhibitor Library I and II, catalogue numbers 539744 and 539745) for their inhibitory activity at 1µM and 10µM against 234 human recombinant kinases using the EMD Millipore KinaseProfilerTM service. A screen profiling the inhibitory activity of 178 commercially available kinase inhibitors at 0.5µM against a panel of 300 recombinant protein kinases using the Reaction Biology Corporation Kinase HotspotSM platform. http://www.millipore.com/techpublications/tech1/pf3036 http://www.reactionbiology.com/webapps/main/pages/kinase.aspx Reference: 3,12 |
 
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: TrkA/TRKA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
References
1. Albaugh P, Fan Y, Mi Y, Sun F, Adrian F, Li N, Jia Y, Sarkisova Y, Kreusch A, Hood T et al.. (2012) Discovery of GNF-5837, a Selective TRK Inhibitor with Efficacy in Rodent Cancer Tumor Models. ACS Med Chem Lett, 3 (2): 140-5. [PMID:24900443]
2. Allen S, Andrews SW, Baer B, Crane Z, Liu W, Watson DJ. (2015) 1-((3s,4r)-4-(3-fluorophenyl)-1-(2-methoxyethyl)pyrrolidin-3-yl)-3-(4-methyl-3-(2-methylpyrimidin-5-yl)-1-phenyl-1h-pyrazol-5-yl)urea as a trka kinase inhibitor. Patent number: WO2015175788A1. Assignee: Array Biopharma Inc.. Priority date: 15/05/2014. Publication date: 19/11/2015.
3. Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. (2011) Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1039-45. [PMID:22037377]
4. Artim SC, Mendrola JM, Lemmon MA. (2012) Assessing the range of kinase autoinhibition mechanisms in the insulin receptor family. Biochem J, 448 (2): 213-20. [PMID:22992069]
5. AstraZeneca. AZD1332. Accessed on 11/09/2014. Modified on 11/09/2014. AstraZeneca.com, http://openinnovation.astrazeneca.com/what-we-offer/compound/azd1332/
6. Bertrand T, Kothe M, Liu J, Dupuy A, Rak A, Berne PF, Davis S, Gladysheva T, Valtre C, Crenne JY et al.. (2012) The crystal structures of TrkA and TrkB suggest key regions for achieving selective inhibition. J Mol Biol, 423 (3): 439-53. [PMID:22902478]
7. Brasca MG, Amboldi N, Ballinari D, Cameron A, Casale E, Cervi G, Colombo M, Colotta F, Croci V, D'Alessio R et al.. (2009) Identification of N,1,4,4-tetramethyl-8-{[4-(4-methylpiperazin-1-yl)phenyl]amino}-4,5-dihydro-1H-pyrazolo[4,3-h]quinazoline-3-carboxamide (PHA-848125), a potent, orally available cyclin dependent kinase inhibitor. J Med Chem, 52 (16): 5152-63. [PMID:19603809]
8. Dahlström M, Madjid N, Nordvall G, Halldin MM, Vazquez-Juarez E, Lindskog M, Sandin J, Winblad B, Eriksdotter M, Forsell P. (2021) Identification of Novel Positive Allosteric Modulators of Neurotrophin Receptors for the Treatment of Cognitive Dysfunction. Cells, 10 (8): 1871. [PMID:34440640]
9. Davies A, Ioannidis S, Lamb M, Su M, Wang T, Zhang H-J. (2013) 9-(pyrazol-3-yl)-9H-purine-2-amine and 3-(pyrazol-3-yl) -3H-imidazo[4,5-B] pyridin-5-amine derivatives and their use for the treatment of cancer. Patent number: US8486966B2. Assignee: AstraZeneca AB. Priority date: 04/05/2007. Publication date: 17/07/2013.
10. Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. (2011) Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1046-51. [PMID:22037378]
11. Drilon A, Ou SI, Cho BC, Kim DW, Lee J, Lin JJ, Zhu VW, Ahn MJ, Camidge DR, Nguyen J et al.. (2018) Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations. Cancer Discov, 8 (10): 1227-1236. [PMID:30093503]
12. Gao Y, Davies SP, Augustin M, Woodward A, Patel UA, Kovelman R, Harvey KJ. (2013) A broad activity screen in support of a chemogenomic map for kinase signalling research and drug discovery. Biochem J, 451 (2): 313-28. [PMID:23398362]
13. Guo J, Xiang S, Wang J, Zhou Y, Wang Z, Zhang Z, Ding K, Lu X. (2022) Discovery of novel TrkA allosteric inhibitors: Structure-based virtual screening, biological evaluation and preliminary SAR studies. Eur J Med Chem, 228: 114022. [PMID:34871843]
14. Haas J, Andrews SW, Jiang Y, Zhang G. (2010) Substituted pyrazolo[1,5-a]pyrimidine compounds as TRK kinase inhibitors. Patent number: WO2010048314 A1. Assignee: Array Biopharma Inc.. Priority date: 22/10/2008. Publication date: 29/04/2010.
15. Horan JC, Tang X, Mente SR, Pelish HE, Shair MD, Tangpeerachaikul A. (2023) Heteroaromatic macrocyclic ether chemotherapeutic agents. Patent number: US11542278. Assignee: Nuvalent, Inc.. Priority date: 29/10/2021. Publication date: 03/01/2023.
16. Hudkins RL, Becknell NC, Zulli AL, Underiner TL, Angeles TS, Aimone LD, Albom MS, Chang H, Miknyoczki SJ, Hunter K et al.. (2012) Synthesis and biological profile of the pan-vascular endothelial growth factor receptor/tyrosine kinase with immunoglobulin and epidermal growth factor-like homology domains 2 (VEGF-R/TIE-2) inhibitor 11-(2-methylpropyl)-12,13-dihydro-2-methyl-8-(pyrimidin-2-ylamino)-4H-indazolo[5,4-a]pyrrolo[3,4-c]carbazol-4-one (CEP-11981): a novel oncology therapeutic agent. J Med Chem, 55 (2): 903-13. [PMID:22148921]
17. Hudkins RL, Johnson NW, Angeles TS, Gessner GW, Mallamo JP. (2007) Synthesis and mixed lineage kinase activity of pyrrolocarbazole and isoindolone analogs of (+)K-252a. J Med Chem, 50 (3): 433-41. [PMID:17266195]
18. Iida H, Fujikawa R, Kozaki R, Harada R, Hosokawa Y, Ogawara KI, Ohno T. (2020) Pharmacokinetic-Pharmacodynamic-Efficacy Modeling of ONO-7579, a Novel Pan-Tropomyosin Receptor Kinase Inhibitor, in a Murine Xenograft Tumor Model. J Pharmacol Exp Ther, 373 (3): 361-369. [PMID:32217770]
19. Ito T, Kinoshita K, Tomizawa M, Shinohara S, Nishii H, Matsushita M, Hattori K, Kohchi Y, Kohchi M, Hayase T et al.. (2022) Discovery of CH7057288 as an Orally Bioavailable, Selective, and Potent pan-TRK Inhibitor. J Med Chem, 65 (18): 12427-12444. [PMID:36066182]
20. Kane JL Jr, Matthews G, Metz M, Kothe M, Liu J, Scholte A. (2015) Tropomyosin-related kinase (Trk) inhibitors. Patent number: US9174986B2. Assignee: Genzyme Corp.. Priority date: 10/12/2013. Publication date: 03/11/2015.
21. Katayama R, Gong B, Togashi N, Miyamoto M, Kiga M, Iwasaki S, Kamai Y, Tominaga Y, Takeda Y, Kagoshima Y et al.. (2019) The new-generation selective ROS1/NTRK inhibitor DS-6051b overcomes crizotinib resistant ROS1-G2032R mutation in preclinical models. Nat Commun, 10 (1): 3604. [PMID:31399568]
22. Kong NX, Zhou C, Zheng Z. (2021) Heterocyclic compound as trk inhibitor. Patent number: US20210147445A1. Assignee: Beijing Innocare Pharma Tech Co Ltd. Priority date: 25/06/2018. Publication date: 20/05/2021.
23. Lee LY, Hernandez D, Rajkhowa T, Smith SC, Raman JR, Nguyen B, Small D, Levis M. (2017) Preclinical studies of gilteritinib, a next-generation FLT3 inhibitor. Blood, 129 (2): 257-260. [PMID:27908881]
24. Li Y, Xiong Y, Zhang G, Zhang L, Yang W, Yang J, Huang L, Qiao Z, Miao Z, Lin G et al.. (2018) Identification of 5-(2,3-Dihydro-1 H-indol-5-yl)-7 H-pyrrolo[2,3- d]pyrimidin-4-amine Derivatives as a New Class of Receptor-Interacting Protein Kinase 1 (RIPK1) Inhibitors, Which Showed Potent Activity in a Tumor Metastasis Model. J Med Chem, 61 (24): 11398-11414. [PMID:30480444]
25. Li Z, Hu Y, Wei W, Liguang D, Xiaowei D, Yanqing Y, Yinghui S, Yongxin H, Yong P, Fansheng K. (2018) Amino pyrazolopyrimidine compound used as neurotrophic factor tyrosine kinase receptor inhibitor. Patent number: WO2018077246A1. Assignee: Chia Tai Tianqing Pharmaceutical Group Co., Ltd., Beijing Sailintai Pharmaceutical Technology Co., Ltd., Lianyungang Runzhong Pharmaceutical Co., Ltd.. Priority date: 27/10/2017. Publication date: 03/05/2018.
26. Manthey CL, Johnson DL, Illig CR, Tuman RW, Zhou Z, Baker JF, Chaikin MA, Donatelli RR, Franks CF, Zeng L et al.. (2009) JNJ-28312141, a novel orally active colony-stimulating factor-1 receptor/FMS-related receptor tyrosine kinase-3 receptor tyrosine kinase inhibitor with potential utility in solid tumors, bone metastases, and acute myeloid leukemia. Mol Cancer Ther, 8 (11): 3151-61. [PMID:19887542]
27. Nanda N, Bilenker JH, Doebele RC, Blake JF, Kolakowski GR, Brandhuber BJ, Andrews SW. (2017) Point mutations in trk inhibitor-resistant cancer and methods relating to the same. Patent number: WO2017075107A1. Assignee: Array Biopharma Inc, Loxo Oncology Inc. Priority date: 26/10/2015. Publication date: 04/05/2017.
28. Pal K, Ciblat S, Albert V, Bruneau-Latour N, Boudreault J. (2021) 5-(2,5-difluorophenyl)pyrrolidin-1-yl)-3-(1h-pyrazol-1-yl)pyrazolo[1,5-a]pyrimidine derivatives and related compounds as trk kinase inhibitors for treating cancer. Patent number: US20210094956A1. Assignee: Pyramid Biosciences Inc. Priority date: 15/12/2017. Publication date: 01/04/2021.
29. Pan BS, Chan GK, Chenard M, Chi A, Davis LJ, Deshmukh SV, Gibbs JB, Gil S, Hang G, Hatch H et al.. (2010) MK-2461, a novel multitargeted kinase inhibitor, preferentially inhibits the activated c-Met receptor. Cancer Res, 70 (4): 1524-33. [PMID:20145145]
30. Patwardhan PP, Ivy KS, Musi E, de Stanchina E, Schwartz GK. (2016) Significant blockade of multiple receptor tyrosine kinases by MGCD516 (Sitravatinib), a novel small molecule inhibitor, shows potent anti-tumor activity in preclinical models of sarcoma. Oncotarget, 7 (4): 4093-109. [PMID:26675259]
31. Roa P, Foglizzo V, Harada G, Repetto M, Kulick A, de Stanchina E, de Marchena M, Auwardt S, Sayed Ahmed S, Bremer NV et al.. (2024) Zurletrectinib is a next-generation TRK inhibitor with strong intracranial activity against NTRK fusion-positive tumours with on-target resistance to first-generation agents. Br J Cancer, 131 (3): 601-610. [PMID:38902532]
32. Robertson AG, Banfield MJ, Allen SJ, Dando JA, Mason GG, Tyler SJ, Bennett GS, Brain SD, Clarke AR, Naylor RL et al.. (2001) Identification and structure of the nerve growth factor binding site on TrkA. Biochem Biophys Res Commun, 282 (1): 131-41. [PMID:11263982]
33. Sun Y, Xue Y, Liu H, Mu S, Sun P, Sun Y, Wang L, Wang H, Wang J, Wu T et al.. (2023) Discovery of CZS-241: A Potent, Selective, and Orally Available Polo-Like Kinase 4 Inhibitor for the Treatment of Chronic Myeloid Leukemia. J Med Chem, 66 (4): 2396-2421. [PMID:36734825]
34. Wang T, Ioannidis S, Almeida L, Block MH, Davies AM, Lamb ML, Scott DA, Su M, Zhang HJ, Alimzhanov M et al.. (2011) In vitro and in vivo evaluation of 6-aminopyrazolyl-pyridine-3-carbonitriles as JAK2 kinase inhibitors. Bioorg Med Chem Lett, 21 (10): 2958-61. [PMID:21493067]
35. Wang T, Lamb ML, Block MH, Davies AM, Han Y, Hoffmann E, Ioannidis S, Josey JA, Liu ZY, Lyne PD et al.. (2012) Discovery of Disubstituted Imidazo[4,5-b]pyridines and Purines as Potent TrkA Inhibitors. ACS Med Chem Lett, 3 (9): 705-9. [PMID:24900538]
36. Wang Z, Zhang Y, Pinkas DM, Fox AE, Luo J, Huang H, Cui S, Xiang Q, Xu T, Xun Q et al.. (2018) Design, Synthesis, and Biological Evaluation of 3-(Imidazo[1,2- a]pyrazin-3-ylethynyl)-4-isopropyl- N-(3-((4-methylpiperazin-1-yl)methyl)-5-(trifluoromethyl)phenyl)benzamide as a Dual Inhibitor of Discoidin Domain Receptors 1 and 2. J Med Chem, 61 (17): 7977-7990. [PMID:30075624]
37. Wodicka LM, Ciceri P, Davis MI, Hunt JP, Floyd M, Salerno S, Hua XH, Ford JM, Armstrong RC, Zarrinkar PP et al.. (2010) Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. Chem Biol, 17 (11): 1241-9. [PMID:21095574]
38. Wu Y, Zhou W, Gong Y, Yue Y, Deng J, Liu Y. (2019) Tropomyosin receptor kinase inhibitor, preparation method therefor and use thereof. Patent number: WO2019233461A1. Assignee: Jiangsu Weikaier Pharmaceutical Technology Co., Ltd.. Priority date: 06/06/2019. Publication date: 12/12/2019.