GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Contents:
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 1 | 766 | 2q24.2 | DPP4 | dipeptidyl peptidase 4 | |
| Mouse | 1 | 760 | 2 35.85 cM | Dpp4 | dipeptidylpeptidase 4 | |
| Rat | 1 | 767 | 3q21 | Dpp4 | dipeptidylpeptidase 4 | |
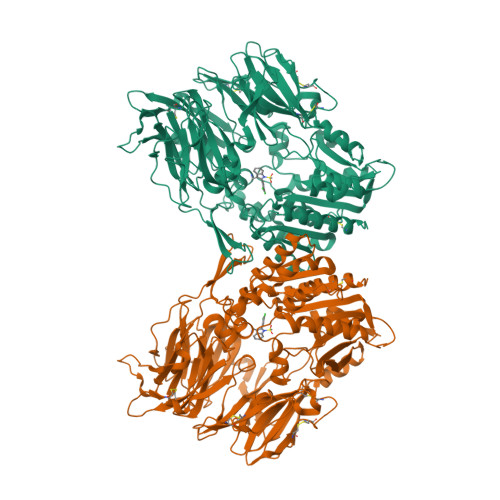
Selected 3D Structures  |
|||||||||||||
|
|
||||||||||||
Enzyme Reaction  |
||||
|
||||
Substrates and Reaction Kinetics  |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antibodies | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
References
1. Benramdane S, De Loose J, Beyens O, Van Rymenant Y, Vliegen G, Augustyns K, De Winter H, De Meester I, Van der Veken P. (2022) Vildagliptin-Derived Dipeptidyl Peptidase 9 (DPP9) Inhibitors: Identification of a DPP8/9-Specific Lead. ChemMedChem, 17 (15): e202200097. [PMID:35760756]
2. Biftu T, Sinha-Roy R, Chen P, Qian X, Feng D, Kuethe JT, Scapin G, Gao YD, Yan Y, Krueger D et al.. (2014) Omarigliptin (MK-3102): a novel long-acting DPP-4 inhibitor for once-weekly treatment of type 2 diabetes. J Med Chem, 57 (8): 3205-12. [PMID:24660890]
3. Connolly BA, Sanford DG, Chiluwal AK, Healey SE, Peters DE, Dimare MT, Wu W, Liu Y, Maw H, Zhou Y et al.. (2008) Dipeptide boronic acid inhibitors of dipeptidyl peptidase IV: determinants of potency and in vivo efficacy and safety. J Med Chem, 51 (19): 6005-13. [PMID:18783201]
4. Davis JA, Singh S, Sethi S, Roy S, Mittra S, Rayasam G, Bansal V, Sattigeri J, Ray A. (2010) Nature of action of Sitagliptin, the dipeptidyl peptidase-IV inhibitor in diabetic animals. Indian J Pharmacol, 42 (4): 229-33. [PMID:20927248]
5. Di Naro AF. (2014) Anti-CD26 Antibodies and Uses Thereof. Patent number: EP2767549 A1. Assignee: Adienne S.A.. Priority date: 19/02/2013. Publication date: 20/08/2014.
6. Eckhardt M, Langkopf E, Mark M, Tadayyon M, Thomas L, Nar H, Pfrengle W, Guth B, Lotz R, Sieger P et al.. (2007) 8-(3-(R)-aminopiperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydropurine-2,6-dione (BI 1356), a highly potent, selective, long-acting, and orally bioavailable DPP-4 inhibitor for the treatment of type 2 diabetes. J Med Chem, 50 (26): 6450-3. [PMID:18052023]
7. Gupta R, Walunj SS, Tokala RK, Parsa KV, Singh SK, Pal M. (2009) Emerging drug candidates of dipeptidyl peptidase IV (DPP IV) inhibitor class for the treatment of Type 2 Diabetes. Curr Drug Targets, 10 (1): 71-87. [PMID:19149538]
8. Jain MR, Joharapurkar AA, Kshirsagar SG, Patel VJ, Bahekar RH, Patel HV, Jadav PA, Patel PR, Desai RC. (2017) ZY15557, a novel, long acting inhibitor of dipeptidyl peptidase-4, for the treatment of Type 2 diabetes mellitus. Br J Pharmacol, 174 (14): 2346-2357. [PMID:28452143]
9. Kato N, Oka M, Murase T, Yoshida M, Sakairi M, Yamashita S, Yasuda Y, Yoshikawa A, Hayashi Y, Makino M et al.. (2011) Discovery and pharmacological characterization of N-[2-({2-[(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl}amino)-2-methylpropyl]-2-methylpyrazolo[1,5-a]pyrimidine-6-carboxamide hydrochloride (anagliptin hydrochloride salt) as a potent and selective DPP-IV inhibitor. Bioorg Med Chem, 19 (23): 7221-7. [PMID:22019046]
10. Li Q, Deng X, Xu YJ, Dong L. (2023) Development of Long-Acting Dipeptidyl Peptidase-4 Inhibitors: Structural Evolution and Long-Acting Determinants. J Med Chem, 66 (17): 11593-11631. [PMID:37647598]
11. Meng W, Brigance RP, Chao HJ, Fura A, Harrity T, Marcinkeviciene J, O'Connor SP, Tamura JK, Xie D, Zhang Y et al.. (2010) Discovery of 6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methylimidazo[1,2-a]pyrimidine-2-carboxamides as potent, selective dipeptidyl peptidase-4 (DPP4) inhibitors. J Med Chem, 53 (15): 5620-8. [PMID:20684603]
12. Tsai TY, Yeh TK, Chen X, Hsu T, Jao YC, Huang CH, Song JS, Huang YC, Chien CH, Chiu JH et al.. (2010) Substituted 4-carboxymethylpyroglutamic acid diamides as potent and selective inhibitors of fibroblast activation protein. J Med Chem, 53 (18): 6572-83. [PMID:20718420]
13. Xiao P, Guo R, Huang S, Cui H, Ye S, Zhang Z. (2014) Discovery of dipeptidyl peptidase IV (DPP4) inhibitors based on a novel indole scaffold. ChinChem Lett, 25 (5): 673-676.
14. Xie H, Zeng L, Zeng S, Lu X, Zhang G, Zhao X, Cheng N, Tu Z, Li Z, Xu H et al.. (2012) Novel pyrrolopyrimidine analogues as potent dipeptidyl peptidase IV inhibitors based on pharmacokinetic property-driven optimization. Eur J Med Chem, 52: 205-12. [PMID:22475866]
15. Yoshida T, Akahoshi F, Sakashita H, Kitajima H, Nakamura M, Sonda S, Takeuchi M, Tanaka Y, Ueda N, Sekiguchi S et al.. (2012) Discovery and preclinical profile of teneligliptin (3-[(2S,4S)-4-[4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)piperazin-1-yl]pyrrolidin-2-ylcarbonyl]thiazolidine): a highly potent, selective, long-lasting and orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Bioorg Med Chem, 20 (19): 5705-19. [PMID:22959556]
16. Yu Q, Wei F. (2010) Beta-amino tetrahydropyrazine, tetrahydropyrimidine and tetrahydropyridine used as dipeptidy peptidase inhibitors for curing or preventing diabetes. Patent number: CN101899047A. Assignee: Shengshi Taike Biopharmaceutical Technology Suzhou Co ltd. Priority date: 26/05/2009. Publication date: 01/12/2010.
17. Zhang C, Ye F, Wang J, He P, Lei M, Huang L, Huang A, Tang P, Lin H, Liao Y et al.. (2020) Design, Synthesis, and Evaluation of a Series of Novel Super Long-Acting DPP-4 Inhibitors for the Treatment of Type 2 Diabetes. J Med Chem, 63 (13): 7108-7126. [PMID:32452679]
How to cite this page
S9: Prolyl oligopeptidase: dipeptidyl peptidase 4. Last modified on 12/06/2025. Accessed on 02/02/2026. IUPHAR/BPS Guide to PHARMACOLOGY, https://www.guidetomalariapharmacology.org/GRAC/ObjectDisplayForward?objectId=1612.