GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
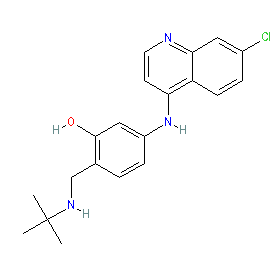
Synonyms: N-tert-Butyl isoquine
Compound class:
Synthetic organic
Comment: GSK369796 is a 4-aminoquinoline antimalarial compound that was synthesised using amodiaquine as the template molecule [3].
The Malaria tab on this ligand page provides additional curator comments of relevance to the Guide to MALARIA PHARMACOLOGY. |
|
|||||||||||||||||||||||||||||||||||
| Guide to Malaria Pharmacology Comments |
| GSK369796 was developed by academics at Liverpool as part of a public-private partnership managed by the Medicines for Malaria Venture (MMV) [3]. This research collaboration, utilised the 4-aminoquinoline scaffold of amodiaquine to design and synthesise derivatives active against both chloroquine-susceptible and chloroquine-resistant P. falciparum strains. Potential Target/Mechanism Of Action: As the precise MOA of GSK369796 is not yet known, we do not have a molecular target for this compound. It is thought that GSK369796 has a similar MOA to chloroquine, killing the malaria parasite by causing a build up of toxic heme by inhibiting the enzyme that normally converts it to non-toxic haemozoin [1]. |