GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
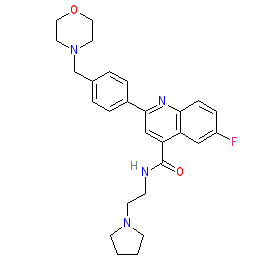
Synonyms: DDD107498 | DDD498 | MMV121
Compound class:
Synthetic organic
Comment: M5717 (formerly DDD498) is a quinoline-4-carboxamide derivative that is the optimised lead developed from a phenotypic screen for novel antimalarial drugs and has advanced to clinical evaluation. The compound was awarded MMV Project of the Year (2014).
The Malaria tab on this ligand page provides additional curator comments of relevance to the Guide to MALARIA PHARMACOLOGY. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| M5717 has completed first-in-human (NCT03261401, last update June 2019) and human challenge (NCT04250363, last update July 2021) Phase 1 clinical trials. |
Mechanism Of Action and Pharmacodynamic Effects  |
| M5717 inhibits P. falciparum protein synthesis, with evidence from genetic experiments indicating P. falciparum elongation factor 2 (PfeEF2) may be the molecular target [1]. eEF2 catalyzes the GTP-dependent translocation of the ribosome along messenger RNA, and is essential for protein synthesis in eukaryotes. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03261401 | First-in-Human Trial of Single Ascending Dose, Multiple Ascending Dose and Malaria Challenge Model in Healthy Subjects | Phase 1 Interventional | Merck KGaA, Darmstadt, Germany | 3 | |
| NCT04250363 | Chemoprophylactic Activity of M5717 in Plasmodium Falciparum Sporozoite (PfSPZ) Challenge Model | Phase 1 Interventional | Merck Healthcare KGaA, Darmstadt, Germany, an affiliate of Merck KGaA, Darmstadt, Germany | ||
Pharmacokinetics  |
| Elimination |
| The mean terminal half-life for elimination ranged from 155 to 193 hours in human volunteers [4]. |