|
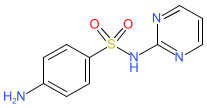
sulfadiazine is an approved drug (FDA (1941), UK (2008))
Compound class:
Synthetic organic
Comment: Sulfadiazine is a sulfonamide with broad-spectrum antimicrobial activity [4]. It is used in the treatment of toxoplasmosis, an infection with the protozoan parasite Toxoplasma gondi that can cause serious complications during pregnancy and in patients with weakened immune systems. Dermatological formulations containing silver sulfadiazine (PubChem CID 441244) are used to prevent and treat wound infections.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Sulfonamides are structural analogues of 4-aminobenzoic acid (pABA) an intermediate in the de novo synthesis of folate by some prokaryotes, lower eukaryotes and plants [2]. The antibacterial MMOA is competitive inhibition of bacterial dihydropteroate synthase (DHPS) resulting in a block of folate biosynthesis [1]. |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com |