GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
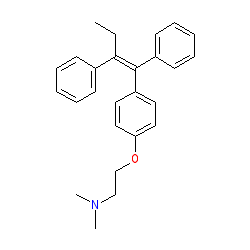
Synonyms: ICI-46474 | Nolvadex® | Soltamox®
tamoxifen is an approved drug (FDA (1977))
Compound class:
Synthetic organic
Comment: Tamoxifen can be classed as a mixed agonist/antagonist of the estrogen -α and -β receptors, as its activity depends on the tissue in which the receptor is expressed. ChEMBL classify this compound as an ERα modulator, while it is described as a selective estrogen receptor modulator by Burris et al. in their 2013 review [1]. Tamoxifen can be considered as a prodrug as its three main metabolites exhibit higher affinity and specificity than tamoxifen at the ERα and ERβ receptors.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| Tamoxifen is one of a number of drugs that are cationic amphiphilic in nature, for which anti-SARS-CoV-2 activity has been identified in drug repurposing screens. Tummino et al. (2021; bioRxiv preprint PMID: 33791693 target="_blank") suggest that this antiviral activity is most likely a result of the drug promoting phospholipidosis via disruption of lipid homeostasis. |
| Enzymes Catalysing Reactions with this Compound as a Substrate or Product | ||||||||
|
||||||||
| Selectivity at GPCRs | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Selectivity at ion channels | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Selectivity at nuclear hormone receptors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Targets where the ligand is described in the comment field | |
| Target | Comment |
| ClC-3 | insensitive to the channel blockers DIDS, NPPB and tamoxifen (10 μM) |
| Maxi Cl- | Maxi Cl- is also activated by G protein-coupled receptors and cell swelling. Tamoxifen and toremifene are examples of triphenylethylene anti-oestrogens |
| Ligand mentioned in the following text fields |
| G protein-coupled estrogen receptor overview |
| 3A. Estrogen receptors comments |
| Glycine receptors comments |
| Maxi chloride channel comments |