GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
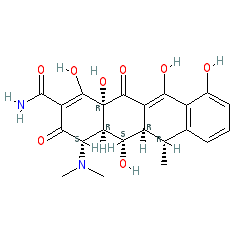
Synonyms: BMY-28689 | Monodox® | Vibramycin®
doxycycline is an approved drug (FDA (1967), UK (2000))
Compound class:
Synthetic organic
Comment: Doxycycline is a tetracycline based antibacterial. It has been repurposed as a low-potency human metalloprotease inhibitor for periodontitis and is also used as an antimalarial therapy.
There are a number of salt forms in PubChem. The chemical structure we show here matches that of the consensus structure in PubChem, listed in the links table below. The DrugBank, ChEBI, ChEMBL and PDB entries for doxycycline show a different structure. Doxycycline is one of the key access group antibacterials on the World Health Organization's Model List of Essential Medicines (link provided in the Classification table, under the Summary tab below). The Malaria tab on this ligand page provides additional curator comments of relevance to the Guide to MALARIA PHARMACOLOGY. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Brandt KD, Mazzuca SA, Katz BP, Lane KA, Buckwalter KA, Yocum DE, Wolfe F, Schnitzer TJ, Moreland LW, Manzi S et al.. (2005)
Effects of doxycycline on progression of osteoarthritis: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum, 52 (7): 2015-25. [PMID:15986343] |
|
2. Dahl EL, Rosenthal PJ. (2007)
Multiple antibiotics exert delayed effects against the Plasmodium falciparum apicoplast. Antimicrob Agents Chemother, 51 (10): 3485-90. [PMID:17698630] |
|
3. Dahl EL, Shock JL, Shenai BR, Gut J, DeRisi JL, Rosenthal PJ. (2006)
Tetracyclines specifically target the apicoplast of the malaria parasite Plasmodium falciparum. Antimicrob Agents Chemother, 50 (9): 3124-31. [PMID:16940111] |
|
4. García RA, Pantazatos DP, Gessner CR, Go KV, Woods Jr VL, Villarreal FJ. (2005)
Molecular interactions between matrilysin and the matrix metalloproteinase inhibitor doxycycline investigated by deuterium exchange mass spectrometry. Mol Pharmacol, 67 (4): 1128-36. [PMID:15665254] |
|
5. Sepper R, Konttinen YT, Ding Y, Takagi M, Sorsa T. (1995)
Human neutrophil collagenase (MMP-8), identified in bronchiectasis BAL fluid, correlates with severity of disease. Chest, 107 (6): 1641-7. [PMID:7781360] |
|
6. Tan KR, Magill AJ, Parise ME, Arguin PM, Centers for Disease Control and Prevention. (2011)
Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. Am J Trop Med Hyg, 84 (4): 517-31. [PMID:21460003] |