GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
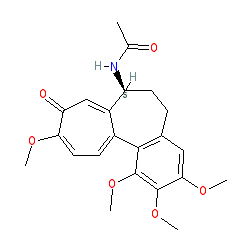
Synonyms: Colcrys® | methoxylated analogue of XD1 | XD25
colchicine is an approved drug (FDA (2009))
Compound class:
Synthetic organic
Comment: Colchicine is a microtubule inhibitor. It is a methoxylated analogue of XD1 (colchicein).
SARS-CoV-2 and COVID-19: There are a number of clinical trials looking at the efficacy of colchicine as a therapy to help treat, and/or prevent the development of, severe symptoms in COVID-19 patients [7]. It has been included in an arm of the UK's RECOVERY trial. Click here to see al clochicine studies on ClinicalTrials.gov [9,11]. Colchicine is predicted to target inflammation and systemic clotting abnormalities that accompany COVID-19 [8,10-11]. This repositioning of colchicine is based both on the drug's established anti-inflammatory action, on its ability to inhibit NLRP3 inflammasome activation, and on experimental evidence that colchicine-induced neutrophil depletion inhibits the release of the endogenous antimicrobial peptide α-defensin-1 (DEFα-1; DEFA1) and reduces thrombus formation in experimental models [2]. Background: contact factors (such as kallikrein and FXIIa) connect inflammation to the activation of coagulation, via promoting the production of DEFα-1 by activated neutrophils. DEFα-1 has pro-thrombotic activity, acting to both stabilise fibrin and thrombus formation and impeding clot resolution by reducing fibrinolysis [2]. In early 2021, preliminary data from the Phase 3 ColCORONA study (NCT04322682) provided some evidence that colchicine reduced hospitalisation (by 25%), progression to mechanical ventilation (by 50%) or death (by 44%) in >4100 confirmed COVID-19 patients, compared to placebo. The drug was given in an outpatient setting. A medRxiv preprint providing more details from the ColCORONA study was posted on 27th January 2021 (DOI: 10.1101/2021.01.26.21250494v1) prior to the peer review process being completed. In contrast a preprint of results from the colchicine arm of the RECOVERY trial (11340 hospitalised COVID-19 patients) reported no clinical benefit compared to standard care by any of the primary or secondary outcome measures (Horby et al., 2021; medRxiv https://doi.org/10.1101/2021.05.18.21257267). Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: colchicine |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Cifuentes M, Schilling B, Ravindra R, Winter J, Janik ME. (2006)
Synthesis and biological evaluation of B-ring modified colchicine and isocolchicine analogs. Bioorg Med Chem Lett, 16 (10): 2761-4. [PMID:16504507] |
|
2. Healy LD, McCarty OJT. (2019)
Contact system sends defensins to the rescue. Blood, 133 (5): 385-386. [PMID:30705047] |
|
3. Lucas X, Wohlwend D, Hügle M, Schmidtkunz K, Gerhardt S, Schüle R, Jung M, Einsle O, Günther S. (2013)
4-Acyl pyrroles: mimicking acetylated lysines in histone code reading. Angew Chem Int Ed Engl, 52 (52): 14055-9. [PMID:24272870] |
|
4. Machu TK. (1998)
Colchicine competitively antagonizes glycine receptors expressed in Xenopus oocytes. Neuropharmacology, 37 (3): 391-6. [PMID:9681937] |
|
5. Martínez GJ, Celermajer DS, Patel S. (2018)
The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis, 269: 262-271. [PMID:29352570] |
|
6. Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. (2010)
The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses, 35 (2): 157-70. [PMID:20022913] |
|
7. Papadopoulos C, Patoulias D, Teperikidis E, Mouselimis D, Tsarouchas A, Toumpourleka M, Boulmpou A, Bakogiannis C, Doumas M, Vassilikos VP. (2020)
Colchicine as a Potential Therapeutic Agent Against Cardiovascular Complications of COVID-19: an Exploratory Review. SN Compr Clin Med, 2 (9): 1419-1429. [PMID:32838182] |
|
8. Reyes AZ, Hu KA, Teperman J, Wampler Muskardin TL, Tardif JC, Shah B, Pillinger MH. (2021)
Anti-inflammatory therapy for COVID-19 infection: the case for colchicine. Ann Rheum Dis, 80 (5): 550-557. [PMID:33293273] |
|
9. Sandhu T, Tieng A, Chilimuri S, Franchin G. (2020)
A Case Control Study to Evaluate the Impact of Colchicine on Patients Admitted to the Hospital with Moderate to Severe COVID-19 Infection. Can J Infect Dis Med Microbiol, 2020: 8865954. [PMID:33133323] |
|
10. Schlesinger N, Firestein BL, Brunetti L. (2020)
Colchicine in COVID-19: an Old Drug, New Use. Curr Pharmacol Rep, 6 (4): 137-145. [PMID:32837853] |
|
11. Vrachatis DA, Giannopoulos GV, Giotaki SG, Raisakis K, Kossyvakis C, Iliodromitis KE, Reimers B, Tousoulis D, Cleman M, Stefanadis C et al.. (2021)
Impact of colchicine on mortality in patients with COVID-19: A meta-analysis. Hellenic J Cardiol, 62 (5): 374-377. [PMID:33421583] |