|
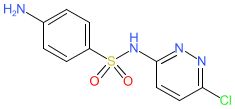
Synonyms: BA-10370 | sulfachloropyridazine | sulphachlorpyridazine
sulfachlorpyridazine is an approved drug (FDA)
Compound class:
Synthetic organic
Comment: Sulfachlorpyridazine is a sulfonamide antibacterial compound.
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Achari A, Somers DO, Champness JN, Bryant PK, Rosemond J, Stammers DK. (1997)
Crystal structure of the anti-bacterial sulfonamide drug target dihydropteroate synthase. Nat Struct Biol, 4 (6): 490-7. [PMID:9187658] |
|
2. Bermingham A, Derrick JP. (2002)
The folic acid biosynthesis pathway in bacteria: evaluation of potential for antibacterial drug discovery. Bioessays, 24 (7): 637-48. [PMID:12111724] |
|
3. NANDA KG, BATTERMAN RC. (1957)
Clinical use of sulfachloropyridazine. Ann N Y Acad Sci, 69 (3): 521-4. [PMID:13488280] |
|
4. Ovung A, Bhattacharyya J. (2021)
Sulfonamide drugs: structure, antibacterial property, toxicity, and biophysical interactions. Biophys Rev, 13 (2): 259-272. [PMID:33936318] |