|
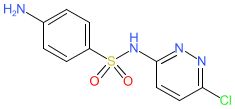
Synonyms: BA-10370 | sulfachloropyridazine | sulphachlorpyridazine
sulfachlorpyridazine is an approved drug (FDA)
Compound class:
Synthetic organic
Comment: Sulfachlorpyridazine is a sulfonamide antibacterial compound.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Sulfachlorpyridazine was used in the treatment of genitourinary tract infections [3]. It was marketed in the US but has been discontinued. It now seems to be used primarily in veterinary medicine. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Sulfonamides are structural analogues of 4-aminobenzoic acid (pABA) an intermediate in the de novo synthesis of folate by some prokaryotes, lower eukaryotes and plants [2]. The antibacterial MMOA is competitive inhibition of bacterial dihydropteroate synthase (DHPS) resulting in a block of folate biosynthesis [1]. |