GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: example E61 [WO2021250648A1] | Paxlovid® (nirmatrelvir + ritonavir) | PF-07321332 | PF07321332
nirmatrelvir is an approved drug (UK MHRA (2021), EMA (2022), FDA (2023))
Compound class:
Synthetic organic
Comment: PF-07321332 (nirmatrelvir) is an oral antiviral clinical lead. It is a covalent inhibitor of SARS-CoV-2 Mpro (main protease, 3CLpro) that binds to cysteine145 within the enzyme's catalytic domain [4-5]. Mpro is essential for coronavirus replication. Inhibiting Mpro actvity blocks replication at an early stage in the virus' life cycle. Due to structural similarities between the Mpro's of other coronavirusus, PF-07321332 offers the potential of pan-coronavirus activity. In vitro it is suggested to inhibit replication of CoV-2 variants including delta and omicron.
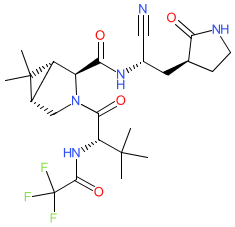
PF-07321332 is structurally similar to ML1000. Prior to peer-reviewed name-to-structure disclosure, we obtained the structure represented here from an online report by Dr Bethany Halford who attended a session by Dafydd Owen (Pfizer) at the ACS Spring 2021 meeting (@beth_halford image on Twitter) which rendered the SMILES string N#C[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H]1N(C[C@H]2[C@@H]1C2(C)C)C(=O)[C@H](C(C)(C)C)NC(=O)C(F)(F)F. PF-07321332's chemical structure was formally disclosed in Owen et al's Science article in November 2021 [3], and this confirmed the structure that was obtained during the ACS meeting. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, Baniecki M, Hendrick VM, Damle B, Simón-Campos A et al.. (2022)
Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med, 386 (15): 1397-1408. [PMID:35172054] |
|
2. Luttens A, Gullberg H, Abdurakhmanov E, Vo DD, Akaberi D, Talibov VO, Nekhotiaeva N, Vangeel L, De Jonghe S, Jochmans D et al.. (2022)
Ultralarge Virtual Screening Identifies SARS-CoV-2 Main Protease Inhibitors with Broad-Spectrum Activity against Coronaviruses. J Am Chem Soc, 144 (7): 2905-2920. [PMID:35142215] |
|
3. Owen DR, Allerton CMN, Anderson AS, Aschenbrenner L, Avery M, Berritt S, Boras B, Cardin RD, Carlo A, Coffman KJ et al.. (2021)
An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science, 374 (6575): 1586-1593. [PMID:34726479] |
|
4. Owen DR, Pettersson MY, Reese MR, Sammons MF, Tuttle JB, Verhoest PR, Wei L, Yang Q, Yang X. (2021)
Nitrile-containing antiviral compounds. Patent number: WO2021250648A1. Assignee: Pfizer Inc.. Priority date: 03/09/2021. Publication date: 16/12/2021. |
|
5. Tuttle JB, Allais C, Allerton CMN, Anderson AS, Arcari JT, Aschenbrenner LM, Avery M, Bellenger J, Berritt S, Boras B et al.. (2025)
Discovery of Nirmatrelvir (PF-07321332): A Potent, Orally Active Inhibitor of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS CoV-2) Main Protease. J Med Chem, [Epub ahead of print]. [PMID:40019854] |