GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
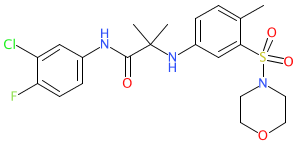
Comment: Compound 28 is the optimized lead from a novel class of antimalarial compounds based on an aminoacetamide scaffold [3].
The Malaria tab on this ligand page provides additional curator comments of relevance to the Guide to MALARIA PHARMACOLOGY. |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Calderón F, Barros D, Bueno JM, Coterón JM, Fernández E, Gamo FJ, Lavandera JL, León ML, Macdonald SJ, Mallo A et al.. (2011)
An Invitation to Open Innovation in Malaria Drug Discovery: 47 Quality Starting Points from the TCAMS. ACS Med Chem Lett, 2 (10): 741-6. [PMID:24900261] |
|
2. Gamo FJ, Sanz LM, Vidal J, de Cozar C, Alvarez E, Lavandera JL, Vanderwall DE, Green DV, Kumar V, Hasan S et al.. (2010)
Thousands of chemical starting points for antimalarial lead identification. Nature, 465 (7296): 305-10. [PMID:20485427] |
|
3. Norcross NR, Wilson C, Baragaña B, Hallyburton I, Osuna-Cabello M, Norval S, Riley J, Fletcher D, Sinden R, Delves M et al.. (2019)
Substituted Aminoacetamides as Novel Leads for Malaria Treatment. ChemMedChem, 14 (14): 1329-1335. [PMID:31188540] |