GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
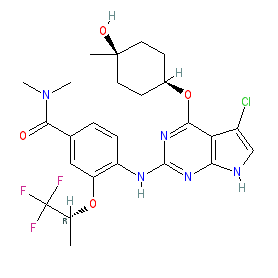
Comment: Compound 20 was rationally designed as a potent, selective and orally active TTK protein kinase inhibitor with single agent efficacy against triple negative breast cancer cells [1]. The pyrrolopyrimidine core of 20 interacts with residues within TTK's ATP binding pocket, and it forms three hydrogen bonds to the hinge region. The ligand/TTK co-crystal structure has been submitted to the PDB and has ID 6N6O.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Riggs JR, Elsner J, Cashion D, Robinson D, Tehrani L, Nagy M, Fultz KE, Krishna Narla R, Peng X, Tran T et al.. (2019)
Design and Optimization Leading to an Orally Active TTK Protein Kinase Inhibitor with Robust Single Agent Efficacy. J Med Chem, 62 (9): 4401-4410. [PMID:30998356] |