GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: Arakoda® | etaquine | Krintafel® | WR238605
tafenoquine is an approved drug (FDA (2018))
Compound class:
Synthetic organic
Comment: Tafenoquine is an 8-aminoquinoline derivative that has potent antimalarial activity and is a synthetic analogue of primaquine.
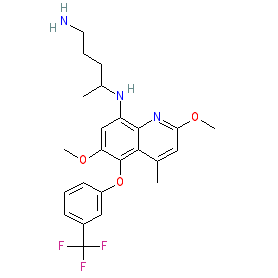
The INN record for tafenoquine indicates that it is a racemic mixture. We show the chemical structure without stereochemistry to represent the mixture. The non-isomeric structure is also represented in the PubChem and ChEMBL entries listed in the links table below, while the two enantiomers forming the racemate are represented by PubChem CID 76969187 and PubChem CID 76969188. The Malaria tab on this ligand page provides additional curator comments of relevance to the Guide to MALARIA PHARMACOLOGY. |
|
|||||||||||||||||||||||||||||||||||
| Guide to Malaria Pharmacology Comments |
| Tafenoquine was identified by researchers at the Walter Reed Army Institute of Research during an antimalarial drug discovery programme that ran from 1963 until the late 1970s. It is, with primaquine, one of only two licensed drugs available for terminal prophylaxis to prevent relapse or delayed-onset clinical presentation of P. vivax malaria. Tafenoquine has a significantly longer elimination half-life than primaquine and is the first drug to be approved for single-dose treatment of relapsing P. vivax malaria. The compound was awarded MMV Project of the Year (2013). Potential Target/Mechanism Of Action: As the precise mechanism of action of tafenoquine is not yet known, we do not have a molecular target for this compound. |
| Target Candidate Profiles | ||||
| Profile | Intended Use | Target Stage | Comment | References |
| TCP-3 | prevention of relapse | hypnozoites | ||