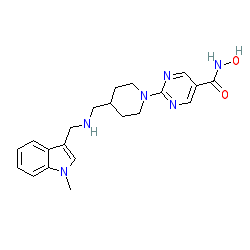
|
Synonyms: JNJ-26481585
Compound class:
Synthetic organic
Comment: Quisinostat is an HDAC inhibitor. The compound has highest potency for HDAC1 and modest potency with HDACs 2, 4, 10, and 11. Quisinostat exhibits >30-fold selectivity against HDACs 3, 5, 8, and 9, with lowest potency for HDACs 6 and 7 [1].
The compound also has antimalarial activity [2]. The Malaria tab on this ligand page provides additional curator comments of relevance to the Guide to MALARIA PHARMACOLOGY. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Guide to Malaria Pharmacology Comments |
| Quisinostat emerged as the most potent antimalarial compound from a phenotypic screen of a library of epigenetic inhibitors [2]. The use of quisinostat as an antimalarial therapy is precluded due to toxicity concerns but it has guided structural optimization studies that have identified promising antimalarial candidates JX21108 [2] and JX35 [5]. Potential Target/Mechanism Of Action: quisinostat is an HDAC inhibitor but we have been unable to find selective affinity data for Plasmodium HDACs. PfHDAC1 is the only Plasmodium HDAC included in the database at present and has been tagged as the target for this ligand for data retrieval purposes. |