GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
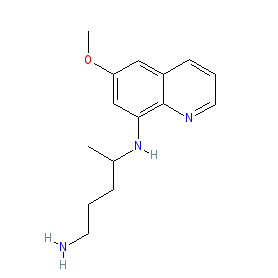
primaquine is an approved drug (FDA (1952))
Compound class:
Synthetic organic
Comment: Primaquine is a synthetic, 8-aminoquinoline derivative that has potent antimalarial activity.
The approved drug is a racemic mixture and we show the chemical structure without stereochemistry to represent the mixture. The non-isomeric structure is also represented in the PubChem, ChEMBL and ChEBI entries listed in the links table below, while the two enantiomers forming the racemate are represented by PubChem CID 3044368 and PubChem CID 3044369. The marketed formulations contain primaquine phosphate (PubChem CID 6135). The Malaria tab on this ligand page provides additional curator comments of relevance to the Guide to MALARIA PHARMACOLOGY. |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Primaquine has a broad spectrum of antimalarial activity and has a number of uses: primary prophylaxis against all Plasmodium species, terminal prophylaxis for P. vivax and P. ovale exposure, and as a radical cure (in combination with an effective blood stage schizonticide) after P. vivax or P. ovale clinical disease [2]. In addition, a recent WHO policy update, recommends the use of primaquine as a gametocytocide to reduce P. falciparum malaria transmission from human to mosquito host. Testing for glucose-6-phosphate dehydrogenase (G6PD) deficiency is required before administration because primaquine causes haemolysis in genetically sensitive individuals [3]. |
Pharmacokinetics  |
| Elimination |
| Primaquine has a terminal half-life for elimination in the 2-12 hour range [2]. |
External links  |
|
For extended ADME data see the following: Drugs.com |