GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
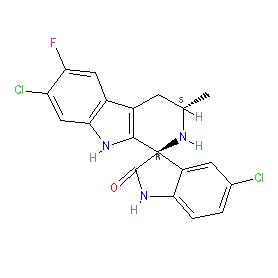
Synonyms: KAE609 | NITD609
Compound class:
Synthetic organic
Comment: Cipargamin is an antimalarial drug candidate, under clinical development. It is the optimised lead for the spiroindolones, a novel class of compounds identified from a phenotypic screen as having antimalarial activity [3,9] and awarded MMV Project of the Year (2009).
The active enantiomer is shown here, with the 1R,3S configuration essential for antimalarial activity [3]. The Malaria tab on this ligand page provides additional curator comments of relevance to the Guide to MALARIA PHARMACOLOGY. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Cipargamin is in clinical development with completion of several Phase 2 clinical trials (NCT01836458, NCT01524341, NCT01860989, NCT03334747) to assess safety and efficacy in patients with uncomplicated P. falciparum or P. vivax malaria. Evaluation of piperaquine as a potential combination therapy partner indicates no significant effect on exposure to either compound and no safety concerns [6]. |
Mechanism Of Action and Pharmacodynamic Effects  |
| The exact mechanism of action of cipargamin is not fully understood but Plasmodium non-SERCA-type Ca2+-transporting P-ATPase (PfATP4) may be a possible target because mutatations in PfATP4 confer resistance to this compound [3]. PfATP4 is thought to regulate Na+ homeostasis and play a role in maintaining the low cytosolic Na+ concentration essential for the Plasmodium parasite to survive during the intraerythrocytic stages of development [5]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT01860989 | A Study to Assess Efficacy, Safety of KAE609 in Adult Patients With Acute Malaria Mono-infection | Phase 2 Interventional | Novartis | ||
| NCT01836458 | A Study to Find the Minimum Inhibitory Concentration of KAE609 in Adult Male Patients With P. Falciparum Monoinfection | Phase 2 Interventional | Novartis | 2 | |
| NCT03334747 | Safety of KAE609 in Adults With Uncomplicated Plasmodium Falciparum Malaria. | Phase 2 Interventional | Novartis | 4 | |
| NCT04675931 | To Evaluate Efficacy, Safety, Tolerability and PK of Intravenous Cipargamin in Participants With Severe Plasmodium Falciparum Malaria | Phase 2 Interventional | Novartis | Not yet recruiting (last updated March 2021). | |
| NCT01524341 | Efficacy, Safety, Tolerability and Pharmacokinetics of KAE609 in Adult Patients With Acute, Uncomplicated Plasmodium Falciparum or Vivax Malaria Mono-infection | Phase 2 Interventional | Novartis | Rapid parasitemia clearance reported in adults with uncomplicated P. falciparum or P. vivax malaria. | 8 |
Pharmacokinetics  |
| Elimination |
| Cipargamin has a mean terminal half-life for elimination of 20.8 hours (range of 11.3 to 37.6 hours), supporting a once-daily oral dosing regimen [8]. |