GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: ACT-128800 | ACT128800 | Compound 8bo [PMID:20446681] | Ponvory®
ponesimod is an approved drug (EMA & FDA (2021))
Compound class:
Synthetic organic
Comment: Ponesimod (ACT-128800) is an orally active sphingosine-1-phosphate (S1P) receptor modulator [1] that was developed as a disease-modifying therapy (DMT) for multiple sclerosis (MS). The compound is reported as a partial S1P4 and S1P5 receptor agonist, with maximum activity 18% and 42% of that induced by the endogenous ligand S1P (at 10μM ACT-128800) [1].
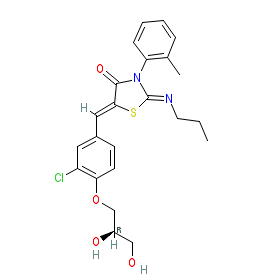
The INN record for ponesimod stipulates the (2R) stereoisomer ((2Z,5Z)-5-{3-chloro-4-[(2R)-2,3-dihydroxypropoxy]phenylmethylidene}-3-(2-methylphenyl)-2-(propylimino)-1,3-thiazolidin-4-one). Selective SIP1 receptor agonists are being developed and investigated for immunomodulatory/immunosuppressant potential in autoimmune diseases. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Ponesimod (ACT-128800) progressed through to late stage development for relapsing multiple sclerosis (MS). Clinical trial NCT02425644 evaluated ponesimod against the dihydroorotate dehydrogenase inhibitor MS drug teriflunomide. In a press release (July 2019) Janssen Pharmaceutical reported positive top-line results from NCT02425644 in patients with relapsing MS. The FDA granted approval in March 2021, as a once-daily oral therapy for relapsing forms of MS. EMA approval followed in May of the same year. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Selective S1P1 receptor agonists appear to act as functional antagonists of lymphocyte movement from the lymph nodes to the circulation. Ponesimod causes the retention of lymphocytes in lymph tissues and thereby decreases circulating lymphocyte numbers, and the number of inflammatory cells available to cross into the central nervous system, where they can cause the myelin damage characteristic of MS. In effect S1P1 receptor agonists act as disease-modifying therapeutics (DMTs). Much drug development effort has focussed on DMTs for MS, and this is reviewed in [2]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02425644 | Oral Ponesimod Versus Teriflunomide In Relapsing MUltiple Sclerosis | Phase 3 Interventional | Actelion | ||