GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: Canef® | Lescol®
fluvastatin is an approved drug (FDA (1993))
Compound class:
Synthetic organic
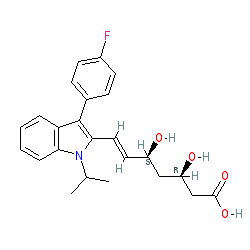
Comment: Fluvastatin is a member of the cholesterol-lowering statin family of drugs. The approved drug is a racemic mixture of a 3R, 5S isomer and a 3S, 5R isomer, and this is supported by the INN document which also indicates a racemate. The 3R, 5S isomer (shown here, and in the PubChem link below) is more pharmacologically active [1,4]. The other isomer is represented by CID 1548972. Representations of the stereochemistry of fluvastatin shown on the resources linked to below may vary from the structure shown here. The majority of the references we cite for our biological activity data do not specify whether the racemate or a specific stereoisomer were used in their experiments.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: fluvastatin |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Fluvastatin is used to treat hypercholesterolaemia and to reduce the risk of developing cardiovascular disease. |