|
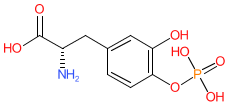
Synonyms: Dopa 4-phosphate
foslevodopa is an approved drug (EMA (2022))
Compound class:
Synthetic organic
Comment: Foslevodopa is a water-soluble levodopa prodrug.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| A combination product (PRODUODOPA®:; ABBV-951) containing foslevodopa and the carbidopa prodrug foscarbidopa was approved by EU EMA, with marketing beginning in early 2024. PRODUODOPA is indicated as a 24-hour infusion therapy for the treatment of advanced Parkinson's disease motor fluctuations. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03781167 | A Study to Evaluate the Safety and Tolerability of ABBV-951 in Subjects With Parkinson's Disease (PD) | Phase 3 Interventional | AbbVie | 1 | |
| NCT04380142 | Study Comparing Continuous Subcutaneous Infusion Of ABBV-951 With Oral Carbidopa/Levodopa Tablets For Treatment Of Motor Fluctuations In Adult Participants With Advanced Parkinson's Disease | Phase 3 Interventional | AbbVie | 2 | |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) |