GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
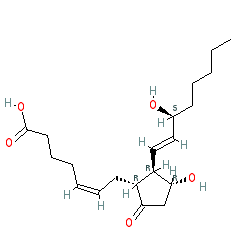
Synonyms: Cervidil® | minprositin E2 | minprostin E2 | Propess® | prostaglandin E2
PGE2 is an approved drug (FDA (1977))
Compound class:
Metabolite
Comment: PGE2 is a the major endogenous prostaglandin. Synthetic PGE2 is known as dinoprostone. PGE2 activates prostanoid family GPCRs.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: prostaglandin e2 |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| Although PGE2 has potent affinity across the EP receptor family we have tagged the EP1 and EP2 receptors as primary drug targets. |
| Natural/Endogenous Targets | ||||||||||
|
||||||||||
| Enzymes Catalysing Reactions with this Compound as a Substrate or Product | ||||||||||||||||||||
|
||||||||||||||||||||
| Transporters Moving this Compound Across a Lipid Membrane | ||||||||||||||||||||
|
||||||||||||||||||||
| Selectivity at GPCRs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Selectivity at ion channels | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information and targets (data relate to human unless otherwise stated) | ||
| Description | Data | Reference |
| Potency order of endogenous ligands at DP1 receptor | PGD2 > PGE1 >> PGE2 > PGF2α > PGI2, thromboxane A2 | |
| Potency order of endogenous ligands at FP receptor | PGF2α > PGD2 > PGE2 > PGI2, thromboxane A2 | |
| Potency order of endogenous ligands at EP4 receptor | PGE2 = PGE1 > PGF2α, PGI2 > PGD2, thromboxane A2 | |
| Potency order of endogenous ligands at DP2 receptor | PGD2 >> PGF2α, PGE2 > PGI2, thromboxane A2 | |
| Potency order of endogenous ligands at TP receptor | thromboxane A2 = PGH2 >> PGD2, PGE2, PGF2α, PGI2 | |
| Potency order of endogenous ligands at EP2 receptor | PGE2 = PGE1 > PGF2α, PGI2 > PGD2, thromboxane A2 | |
| Potency order of endogenous ligands at EP1 receptor | PGE2 > PGE1 > PGF2α, PGI2 > PGD2, thromboxane A2 | |
| Potency order of endogenous ligands at EP3 receptor | PGE2, PGE1 > PGF2α, PGI2 > PGD2, thromboxane A2 | |
| Targets where the ligand is described in the comment field | |
| Target | Comment |
| ABCC4 | Although reported to facilitate cellular cyclic nucleotide export, this role has been questioned [4]; reported to export prostaglandins in a manner sensitive to NSAIDS [17]. A common binding site accommodates substrates including PGE1, PGE2 and dehydroepiandrosterone sulphate (DHEAS) [16]. |
| mPGES2 | Heme-free mPGES2 catalyses the isomerisation of prostaglandin H2 (PGE2) to prostaglandin E2 (PGE2) in the presence of glutathione (GSH); in contrast heme-bound mPGES2 catalyses the degradation of PGH2 to 12-hydroxyheptadecatrienoic acid (HHT) and malondialdehyde (MDA) [24,28-29]. In colon cancer cells artemisinin has been reported to bind reversibly to mPGES2 (occupying the PGH2 site), with the effect of blocking PGE2 biosynthesis and inhibiting cell proliferation [6]. |
| Ligand mentioned in the following text fields |
| Prostaglandin synthases overview |
| Prostanoid receptors overview |