GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: Arakoda® | etaquine | Krintafel® | WR238605
tafenoquine is an approved drug (FDA (2018))
Compound class:
Synthetic organic
Comment: Tafenoquine is an 8-aminoquinoline derivative that has potent antimalarial activity and is a synthetic analogue of primaquine.
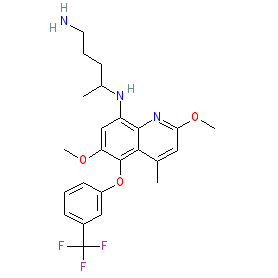
The INN record for tafenoquine indicates that it is a racemic mixture. We show the chemical structure without stereochemistry to represent the mixture. The non-isomeric structure is also represented in the PubChem and ChEMBL entries listed in the links table below, while the two enantiomers forming the racemate are represented by PubChem CID 76969187 and PubChem CID 76969188. The Malaria tab on this ligand page provides additional curator comments of relevance to the Guide to MALARIA PHARMACOLOGY. |
|
|||||||||||||||||||||||||||||||||||
Classification  |
|
| Compound class | Synthetic organic |
| Ligand families/groups | Antimalarial ligands |
| Approved drug? | Yes. US FDA (2018) |
IUPAC Name  |
| N-[2,6-dimethoxy-4-methyl-5-[3-(trifluoromethyl)phenoxy]quinolin-8-yl]pentane-1,4-diamine |
International Nonproprietary Names  |
|
| INN number | INN |
| 7835 | tafenoquine |
Synonyms  |
| Arakoda® | etaquine | Krintafel® | WR238605 |
Database Links  |
|
| CAS Registry No. | 106635-80-7 (source: Scifinder) |
| ChEMBL Ligand | CHEMBL298470 |
| DrugCentral Ligand | 3578 |
| GtoPdb PubChem SID | 374883823 |
| PubChem CID | 115358 |
| Search Google for chemical match using the InChIKey | LBHLFPGPEGDCJG-UHFFFAOYSA-N |
| Search Google for chemicals with the same backbone | LBHLFPGPEGDCJG |
| Search PubMed clinical trials | tafenoquine |
| Search PubMed titles | tafenoquine |
| Search PubMed titles/abstracts | tafenoquine |
| UniChem Compound Search for chemical match using the InChIKey | LBHLFPGPEGDCJG-UHFFFAOYSA-N |
| UniChem Connectivity Search for chemical match using the InChIKey | LBHLFPGPEGDCJG-UHFFFAOYSA-N |
| Wikipedia | Tafenoquine |