GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
Comment: Structural studies have shown that thalidomide class immunomodulatory drugs bind in a shallow hydrophobic pocket on the surface of cereblon, that is mediated by a glutarimide ring of the ligand. Binding of small molecules to cereblon can alter its substrate specificity [1]. Thalidomide and its related drugs enhance recruitment of Ikaros and Aiolos to the E3 ubiquitin ligase complex. CC-885 has a different mechanism of action compared to thalidomide, lenalidomide and pomalidomide. The anti-tumour activity of CC-885 is mediated through the cereblon-dependent ubiquitination and degradation of the translation termination factor GSPT1. Patient-derived acute myeloid leukaemia tumour cells exhibit high sensitivity to CC-885, indicating the clinical potential of this mechanism [2].
|
|
|||||||||||||||||||||||||||||||||||
Classification  |
|
| Compound class | Synthetic organic |
IUPAC Name  |
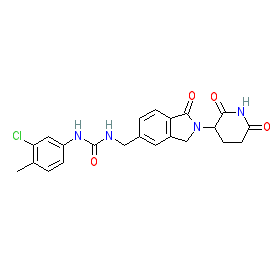
| 3-(3-chloro-4-methylphenyl)-1-[[2-(2,6-dioxopiperidin-3-yl)-1-oxo-3H-isoindol-5-yl]methyl]urea |
Database Links  |
|
| GtoPdb PubChem SID | 340590211 |
| PubChem CID | 24788636 |
| Search Google for chemical match using the InChIKey | DOEVCIHTTTYVCC-UHFFFAOYSA-N |
| Search Google for chemicals with the same backbone | DOEVCIHTTTYVCC |
| UniChem Compound Search for chemical match using the InChIKey | DOEVCIHTTTYVCC-UHFFFAOYSA-N |
| UniChem Connectivity Search for chemical match using the InChIKey | DOEVCIHTTTYVCC-UHFFFAOYSA-N |
Product suppliers
View disclaimer
Sponsored products disclaimer
Product supplier links are provided as a service to assist in identifying commercial suppliers of reagents that are mentioned on the IUPHAR/BPS Guide to PHARMACOLOGY database website, and do not imply their endorsement by NC-IUPHAR.
Links are provided in return for sponsorship, used to fund improvements to this database. The sponsorship account is managed and audited by the University of Edinburgh, a charitable body registered in Scotland, SC005336. If you are interested in sponsoring the database, please contact us.
✖
Sponsored products disclaimer
Product supplier links are provided as a service to assist in identifying commercial suppliers of reagents that are mentioned on the IUPHAR/BPS Guide to PHARMACOLOGY database website, and do not imply their endorsement by NC-IUPHAR.
Links are provided in return for sponsorship, used to fund improvements to this database. The sponsorship account is managed and audited by the University of Edinburgh, a charitable body registered in Scotland, SC005336. If you are interested in sponsoring the database, please contact us.
✖|
CC-885 (links to external site)
Cat. No. HY-101488 |