GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
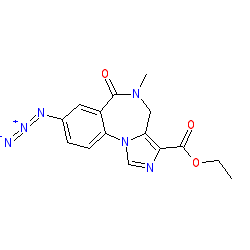
Compound class:
Synthetic organic
|
|
|||||||||||||||||||||||||||||||||||
| Selectivity at ion channels | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Targets where the ligand is described in the comment field | |
| Target | Comment |
| GABAA receptor α6 subunit | Diazepam and flunitrazepam are not active at channels containing this subunit. Zn2+ is an endogenous allosteric regulator and causes potent inhibition of receptors formed from binary combinations of α and β subunits, incorporation of a δ or γ subunit causes a modest, or pronounced, reduction in inhibitory potency, respectively [1]. [3H]Ro154513 selectively labels α6-subunit and α4-subunit-containing receptors in the presence of a saturating concentration of a 'classical' benzodiazepine (e.g. diazepam). Sieghart et al. (2022) provides a review of the pharmacology of α6-containing GABAA receptors. |
| GABAA receptor α4 subunit | Diazepam and flunitrazepam are not active at this subunit. Zn2+ is an endogenous allosteric regulator and causes potent inhibition of receptors formed from binary combinations of α and β subunits, incorporation of a δ or γ subunit causes a modest, or pronounced, reduction in inhibitory potency, respectively [1]. [3H]Ro154513 labels α4βγ2 and α6βγ2 receptors in the presence of a saturating concentration of a 'classical' benzodiazepine (e.g. diazepam). |