GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
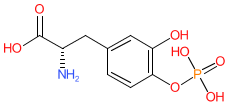
Synonyms: Dopa 4-phosphate
foslevodopa is an approved drug (EMA (2022))
Compound class:
Synthetic organic
Comment: Foslevodopa is a water-soluble levodopa prodrug.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| A combination product (Produodopa®:; ABBV-951) containing foslevodopa and the carbidopa prodrug foscarbidopa was approved by EU EMA and UK MHRA (in the third quarter of 2022), with clinical use beginning in early 2024. Produodopa is indicated as a 24-hour subcutaneous infusion therapy for the treatment of advanced Parkinson's disease motor fluctuations. This same comnination product was approved by the FDA in October 2024 (as Vyalev®). |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04380142 | Study Comparing Continuous Subcutaneous Infusion Of ABBV-951 With Oral Carbidopa/Levodopa Tablets For Treatment Of Motor Fluctuations In Adult Participants With Advanced Parkinson's Disease | Phase 3 Interventional | AbbVie | 2 | |
| NCT03781167 | A Study to Evaluate the Safety and Tolerability of ABBV-951 in Subjects With Parkinson's Disease (PD) | Phase 3 Interventional | AbbVie | 1 | |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) |