GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: example E61 [WO2021250648A1] | Paxlovid® (nirmatrelvir + ritonavir) | PF-07321332 | PF07321332
nirmatrelvir is an approved drug (UK MHRA (2021), EMA (2022), FDA (2023))
Compound class:
Synthetic organic
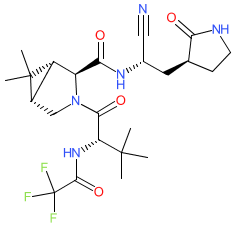
Comment: PF-07321332 (nirmatrelvir) is an oral antiviral clinical lead. It is a covalent inhibitor of SARS-CoV-2 Mpro (main protease, 3CLpro) that binds to cysteine145 within the enzyme's catalytic domain [5-6]. Mpro is essential for coronavirus replication. Inhibiting Mpro actvity blocks replication at an early stage in the virus' life cycle. Due to structural similarities between the Mpro's of other coronavirusus, PF-07321332 offers the potential of pan-coronavirus activity. In vitro it is suggested to inhibit replication of CoV-2 variants including delta and omicron.
PF-07321332 is structurally similar to ML1000. Prior to peer-reviewed name-to-structure disclosure, we obtained the structure represented here from an online report by Dr Bethany Halford who attended a session by Dafydd Owen (Pfizer) at the ACS Spring 2021 meeting (@beth_halford image on Twitter) which rendered the SMILES string N#C[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H]1N(C[C@H]2[C@@H]1C2(C)C)C(=O)[C@H](C(C)(C)C)NC(=O)C(F)(F)F. PF-07321332's chemical structure was formally disclosed in Owen et al's Science article in November 2021 [4], and this confirmed the structure that was obtained during the ACS meeting. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Nirmatrelvir (PF-07321332) was progressed to clinical trial to determine safety and efficacy as a treatment for COVID-19. Phase 1 (NCT04756531) results were published in November 2021 [4]. Click here to link to ClinicalTrials.gov's full list of PF-07321332 studies. A few days after the Phase 1 results were published, Pfizer announced (via press release) that interim analysis from their Phase 3 EPIC-HR study (NCT04960202), indicated that PF-07321332 (in combination with ritonavir) reduced the risk of hospitalisation or death by almost 90% in patients who were at high risk of progressing to severe illness, and who were treated within 3 days of symptom onset. Final results of NCT04960202 were formally published in February 2022, confirming the preliminary efficacy [2]. This study found that PF-07321332/ritonavir treatment reduced viral load in trial participants (compared to placebo), which suggests the potential for reduced transmission. Ritonavir is included in the dosing regimen to inhibit CYP450-mediated metabolic clearance of PF-07321332. The UK's MHRA approved Paxlovid in December 2021, and it was granted conditional marketing authorisation by the EMA on 28th Jan. 2022. The FDA approved Paxlovid in May 2023. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04756531 | STUDY OF PF-07321332 IN HEALTHY PARTICIPANTS | Phase 1 Interventional | Pfizer | 4 | |
| NCT04960202 | A Study of PF-07321332/Ritonavir in Nonhospitalized High Risk Adult Participants With COVID-19 | Phase 2/Phase 3 Interventional | Pfizer | In November 2021 Pfizer announced that PF-07321332 (in combination with |
|
| NCT05965726 | RECOVER-VITAL: Platform Protocol, Appendix to Measure the Effects of Paxlovid on Long COVID Symptoms | Phase 2 Interventional | Duke University | RECOVER-VITAL study is designed to evaluate efficacy as treatment for established long COVID. | |
| NCT05823896 | ImPROving Quality of LIFe in the Long COVID Patient | Phase 2 Interventional | Karolinska Institutet | ||
| NCT05576662 | Paxlovid for Treatment of Long Covid | Phase 2 Interventional | Stanford University | STOP-PASC study is designed to evaluate efficacy as treatment for established long COVID. | |
| NCT05668091 | A Decentralized, Randomized Phase 2 Efficacy and Safety Study of Nirmatrelvir/Ritonavir in Adults with Long COVID. | Phase 2 Interventional | Yale University | ||