GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Nuclear receptor related 1
Target id: 630
Nomenclature: Nuclear receptor related 1
Systematic Nomenclature: NR4A2
Contents:
- Gene and Protein Information
- Previous and Unofficial Names
- Database Links
- Selected 3D Structures
- Natural/Endogenous Ligands
- Agonists
- DNA Binding
- Co-binding Partners
- Main Target Genes
- Tissue Distribution
- Physiological Consequences of Altering Gene Expression
- Phenotypes, Alleles and Disease Models
- Clinically-Relevant Mutations and Pathophysiology
- Biologically Significant Variants
- References
- How to cite this page
Gene and Protein Information  |
|||||
| Species | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 598 | 2q22-q23 | NR4A2 | nuclear receptor subfamily 4 group A member 2 | 15 |
| Mouse | 598 | 2 31.66 cM | Nr4a2 | nuclear receptor subfamily 4, group A, member 2 | 9 |
| Rat | 598 | 3q21 | Nr4a2 | nuclear receptor subfamily 4, group A, member 2 | 20 |
Database Links  |
|
| Alphafold | P43354 (Hs), Q06219 (Mm), Q07917 (Rn) |
| CATH/Gene3D | 3.30.50.10 |
| ChEMBL Target | CHEMBL5002 (Hs), CHEMBL3879839 (Mm), CHEMBL4523278 (Rn) |
| Ensembl Gene | ENSG00000153234 (Hs), ENSMUSG00000026826 (Mm), ENSRNOG00000005600 (Rn) |
| Entrez Gene | 4929 (Hs), 18227 (Mm), 54278 (Rn) |
| Human Protein Atlas | ENSG00000153234 (Hs) |
| KEGG Gene | hsa:4929 (Hs), mmu:18227 (Mm), rno:54278 (Rn) |
| OMIM | 601828 (Hs) |
| Orphanet | ORPHA159799 (Hs) |
| Pharos | P43354 (Hs) |
| RefSeq Nucleotide | NM_006186 (Hs), NM_013613 (Mm), NM_001139509 (Mm), NM_019328 (Rn) |
| RefSeq Protein | NP_006177 (Hs), NP_038641 (Mm), NP_062201 (Rn) |
| UniProtKB | P43354 (Hs), Q06219 (Mm), Q07917 (Rn) |
| Wikipedia | NR4A2 (Hs) |
Selected 3D Structures  |
|||||||||||
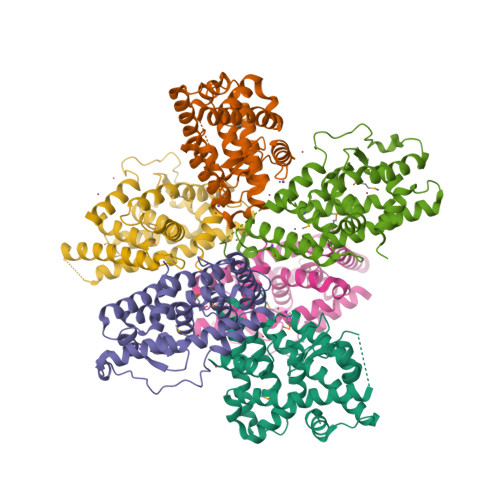
|
|
||||||||||
Natural/Endogenous Ligands  |
| Comments: Orphan |
Download all structure-activity data for this target as a CSV file 
Agonists  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Agonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NR4A2 (NURR) is the first nuclear receptor described that lacks a bona fide ligand binding pocket in its LBD. It is therefore the first unambiguously orphan nuclear receptor [24]. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Co-binding Partners  |
|||
| Name | Interaction | Effect | Reference |
| Nerve Growth factor IB | Physical, Functional | DNA binding | 12 |
| Neuron-derived orphan receptor 1 | Physical, Functional | DNA binding | 12 |
| Retinoid X receptor-α | Physical, Functional | DNA binding | 16,26 |
| CDKN1C (p57KIP2) | Physical, Functional | specify NR4A3/p57kip2 interaction seems to inhibit NR4A2 transcriptional activity | 7 |
| PIASγ | Physical, Functional | represses Nurr1 transcriptional activity | 3 |
Main Target Genes  |
|||||
| Name | Species | Effect | Technique | Comments | References |
| Spp1 | Mouse | Activated | Transient transfection, EMSA, Other | The activation of the OPN promoter is mediated by the monomeric form of Nurr1, required direct binding of Nurr1 to the OPN promoter, and is dependent on the amino-terminal transactivation function-1. The OPN promoter is also regulated by vitamin D receptor and estrogen-related receptors. Nurr1 and vitamin D activate the OPN promoter in a synergistic fashion, whereas Nurr1-mediated transactivation of the OPN promoter is repressed by estrogen-related receptors. | 8 |
| Bglap | Rat | Activated | ChIP, Transient transfection, EMSA | NR4A2 positively regulate the osteocalcin promoter as a monomer. | 17 |
| Th | Mouse | Activated | ChIP, Transient transfection, EMSA, Footprint | NR4A2 stimulates TH promoter activity in mouse and rat but not in human | 18 |
| Nrp1 | Mouse | Activated | ChIP, Transient transfection | 5 | |
Tissue Distribution 
|
||||||||
|
||||||||
| Tissue Distribution Comments | ||||||||
| The NR4A2 is expressed as a 3.5 kb transcript and exhibit a strong inducibility, for example by membrane depolarisation in PC12 cells.The three NR4A subfamily members are expressed in a complex pattern in the nervous system where they are induced as part of the immediate early response to stimuli such as growth factors, membrane depolarisation and seizure. Their expression pattern outside the nervous system is quite large. NR4A2 is expressed more specifically in mesencephalic dopaminergic neurones of the ventral tegmental area and of the substantia nigra. Outside of the CNS, NR4A2 is expressed in the adult liver as well as in pituitary, thymus and osteoblasts. It is important to notice that NR4A2 is constitutively expressed in various region of the CNS and quickly induced as an immediate early gene in numerous peripheric tissus and in the CNS in response to various stimuli. (NB: similar patterns are seen in rodents). | ||||||||
Physiological Consequences of Altering Gene Expression 
|
||||||||||
|
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
Biologically Significant Variants 
|
||||||||
|
References
1. Bandoh S, Tsukada T, Maruyama K, Ohkura N, Yamaguchi K. (1997) Differential expression of NGFI-B and RNR-1 genes in various tissues and developing brain of the rat: comparative study by quantitative reverse transcription-polymerase chain reaction. J Neuroendocrinol, 9 (1): 3-8. [PMID:9023733]
2. Castillo SO, Baffi JS, Palkovits M, Goldstein DS, Kopin IJ, Witta J, Magnuson MA, Nikodem VM. (1998) Dopamine biosynthesis is selectively abolished in substantia nigra/ventral tegmental area but not in hypothalamic neurons in mice with targeted disruption of the Nurr1 gene. Mol Cell Neurosci, 11 (1-2): 36-46. [PMID:9608532]
3. Galleguillos D, Vecchiola A, Fuentealba JA, Ojeda V, Alvarez K, Gómez A, Andrés ME. (2004) PIASgamma represses the transcriptional activation induced by the nuclear receptor Nurr1. J Biol Chem, 279 (3): 2005-11. [PMID:14559918]
4. Hering R, Petrovic S, Mietz EM, Holzmann C, Berg D, Bauer P, Woitalla D, Müller T, Berger K, Krüger R, Riess O. (2004) Extended mutation analysis and association studies of Nurr1 (NR4A2) in Parkinson disease. Neurology, 62 (7): 1231-2. [PMID:15079038]
5. Hermanson E, Borgius L, Bergsland M, Joodmardi E, Perlmann T. (2006) Neuropilin1 is a direct downstream target of Nurr1 in the developing brain stem. J Neurochem, 97 (5): 1403-11. [PMID:16638018]
6. Ibáñez P, Lohmann E, Pollak P, Durif F, Tranchant C, Agid Y, Dürr A, Brice A, French Parkinson's Disease Genetics Study Group. (2004) Absence of NR4A2 exon 1 mutations in 108 families with autosomal dominant Parkinson disease. Neurology, 62 (11): 2133-4. [PMID:15184637]
7. Joseph B, Wallén-Mackenzie A, Benoit G, Murata T, Joodmardi E, Okret S, Perlmann T. (2003) p57(Kip2) cooperates with Nurr1 in developing dopamine cells. Proc Natl Acad Sci USA, 100 (26): 15619-24. [PMID:14671317]
8. Lammi J, Huppunen J, Aarnisalo P. (2004) Regulation of the osteopontin gene by the orphan nuclear receptor NURR1 in osteoblasts. Mol Endocrinol, 18 (6): 1546-57. [PMID:14988426]
9. Law SW, Conneely OM, DeMayo FJ, O'Malley BW. (1992) Identification of a new brain-specific transcription factor, NURR1. Mol Endocrinol, 6 (12): 2129-35. [PMID:1491694]
10. Le WD, Xu P, Jankovic J, Jiang H, Appel SH, Smith RG, Vassilatis DK. (2003) Mutations in NR4A2 associated with familial Parkinson disease. Nat Genet, 33 (1): 85-9. [PMID:12496759]
11. Mages HW, Rilke O, Bravo R, Senger G, Kroczek RA. (1994) NOT, a human immediate-early response gene closely related to the steroid/thyroid hormone receptor NAK1/TR3. Mol Endocrinol, 8 (11): 1583-91. [PMID:7877627]
12. Maira M, Martens C, Philips A, Drouin J. (1999) Heterodimerization between members of the Nur subfamily of orphan nuclear receptors as a novel mechanism for gene activation. Mol Cell Biol, 19 (11): 7549-57. [PMID:10523643]
13. Nsegbe E, Wallén-Mackenzie A, Dauger S, Roux JC, Shvarev Y, Lagercrantz H, Perlmann T, Herlenius E. (2004) Congenital hypoventilation and impaired hypoxic response in Nurr1 mutant mice. J Physiol (Lond.), 556 (Pt 1): 43-59. [PMID:14742729]
14. Ohkura N, Hosono T, Maruyama K, Tsukada T, Yamaguchi K. (1999) An isoform of Nurr1 functions as a negative inhibitor of the NGFI-B family signaling. Biochim Biophys Acta, 1444 (1): 69-79. [PMID:9931442]
15. Okabe T, Takayanagi R, Imasaki K, Haji M, Nawata H, Watanabe T. (1995) cDNA cloning of a NGFI-B/nur77-related transcription factor from an apoptotic human T cell line. J Immunol, 154 (8): 3871-9. [PMID:7706727]
16. Perlmann T, Jansson L. (1995) A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev, 9 (7): 769-82. [PMID:7705655]
17. Pirih FQ, Tang A, Ozkurt IC, Nervina JM, Tetradis S. (2004) Nuclear orphan receptor Nurr1 directly transactivates the osteocalcin gene in osteoblasts. J Biol Chem, 279 (51): 53167-74. [PMID:15485875]
18. Sakurada K, Ohshima-Sakurada M, Palmer TD, Gage FH. (1999) Nurr1, an orphan nuclear receptor, is a transcriptional activator of endogenous tyrosine hydroxylase in neural progenitor cells derived from the adult brain. Development, 126 (18): 4017-26. [PMID:10457011]
19. Saucedo-Cardenas O, Quintana-Hau JD, Le WD, Smidt MP, Cox JJ, De Mayo F, Burbach JP, Conneely OM. (1998) Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci USA, 95 (7): 4013-8. [PMID:9520484]
20. Scearce LM, Laz TM, Hazel TG, Lau LF, Taub R. (1993) RNR-1, a nuclear receptor in the NGFI-B/Nur77 family that is rapidly induced in regenerating liver. J Biol Chem, 268 (12): 8855-61. [PMID:8473329]
21. Stiller T, Merk D. (2023) Exploring Fatty Acid Mimetics as NR4A Ligands. J Med Chem, 66 (22): 15362-15369. [PMID:37918435]
22. Vietor J, Gege C, Stiller T, Busch R, Schallmayer E, Kohlhof H, Höfner G, Pabel J, Marschner JA, Merk D. (2023) Development of a Potent Nurr1 Agonist Tool for In Vivo Applications. J Med Chem,. DOI: 10.1021/acs.jmedchem.3c00415
23. Wallén A A, Castro DS, Zetterström RH, Karlén M, Olson L, Ericson J, Perlmann T. (2001) Orphan nuclear receptor Nurr1 is essential for Ret expression in midbrain dopamine neurons and in the brain stem. Mol Cell Neurosci, 18 (6): 649-63. [PMID:11749040]
24. Wang Z, Benoit G, Liu J, Prasad S, Aarnisalo P, Liu X, Xu H, Walker NP, Perlmann T. (2003) Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature, 423 (6939): 555-60. [PMID:12774125]
25. Zetterström RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T. (1997) Dopamine neuron agenesis in Nurr1-deficient mice. Science, 276 (5310): 248-50. [PMID:9092472]
26. Zetterström RH, Solomin L, Mitsiadis T, Olson L, Perlmann T. (1996) Retinoid X receptor heterodimerization and developmental expression distinguish the orphan nuclear receptors NGFI-B, Nurr1, and Nor1. Mol Endocrinol, 10 (12): 1656-66. [PMID:8961274]
27. Zetterström RH, Williams R, Perlmann T, Olson L. (1996) Cellular expression of the immediate early transcription factors Nurr1 and NGFI-B suggests a gene regulatory role in several brain regions including the nigrostriatal dopamine system. Brain Res Mol Brain Res, 41 (1-2): 111-20. [PMID:8883941]
How to cite this page
4A. Nerve growth factor IB-like receptors: Nuclear receptor related 1. Last modified on 03/11/2023. Accessed on 07/02/2026. IUPHAR/BPS Guide to PHARMACOLOGY, https://www.guidetomalariapharmacology.org/GRAC/ObjectDisplayForward?objectId=630.









