GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Contents:
- Gene and Protein Information
- Previous and Unofficial Names
- Database Links
- Selected 3D Structures
- Natural/Endogenous Ligands
- Agonists
- Antagonists
- Allosteric Modulators
- Immunopharmacology Comments
- Immuno Cell Type Associations
- Transduction Mechanisms
- Tissue Distribution
- Expression Datasets
- Functional Assays
- Physiological Functions
- Physiological Consequences of Altering Gene Expression
- Phenotypes, Alleles and Disease Models
- Biologically Significant Variants
- General Comments
- References
- Contributors
- How to cite this page
Gene and Protein Information  |
||||||
| class A G protein-coupled receptor | ||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 7 | 472 | 6q15 | CNR1 | cannabinoid receptor 1 | 29,38 |
| Mouse | 7 | 473 | 4 16.28 cM | Cnr1 | cannabinoid receptor 1 | 9 |
| Rat | 7 | 473 | 5q21 | Cnr1 | cannabinoid receptor 1 | 68 |
Previous and Unofficial Names  |
|
| Central cannabinoid receptor | Neuronal cannabinoid receptor | THC receptor | CB-R | Cann6 [90] | SKR6R | CB1R | Cann7 | |
Database Links  |
|
| Specialist databases | |
| GPCRdb | cnr1_human (Hs), cnr1_mouse (Mm), cnr1_rat (Rn) |
| Other databases | |
| Alphafold | P21554 (Hs), P47746 (Mm), P20272 (Rn) |
| ChEMBL Target | CHEMBL218 (Hs), CHEMBL3037 (Mm), CHEMBL3571 (Rn) |
| DrugBank Target | P21554 (Hs) |
| Ensembl Gene | ENSG00000118432 (Hs), ENSMUSG00000044288 (Mm), ENSRNOG00000008223 (Rn) |
| Entrez Gene | 1268 (Hs), 12801 (Mm), 25248 (Rn) |
| Human Protein Atlas | ENSG00000118432 (Hs) |
| KEGG Gene | hsa:1268 (Hs), mmu:12801 (Mm), rno:25248 (Rn) |
| OMIM | 114610 (Hs) |
| Pharos | P21554 (Hs) |
| RefSeq Nucleotide | NM_016083 (Hs), NM_007726 (Mm), NM_012784 (Rn) |
| RefSeq Protein | NP_057167 (Hs), NP_031752 (Mm), NP_036916 (Rn) |
| SynPHARM | 84430 (in complex with AM11542) |
| UniProtKB | P21554 (Hs), P47746 (Mm), P20272 (Rn) |
| Wikipedia | CNR1 (Hs) |
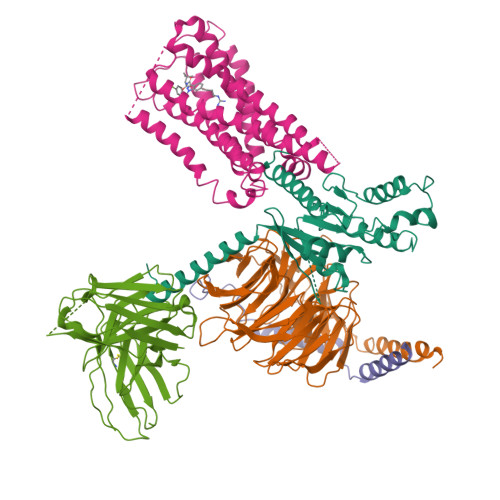
Selected 3D Structures  |
|||||||||||||
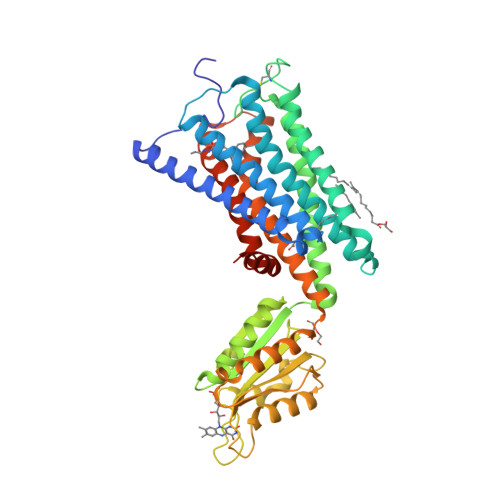
|
|
||||||||||||
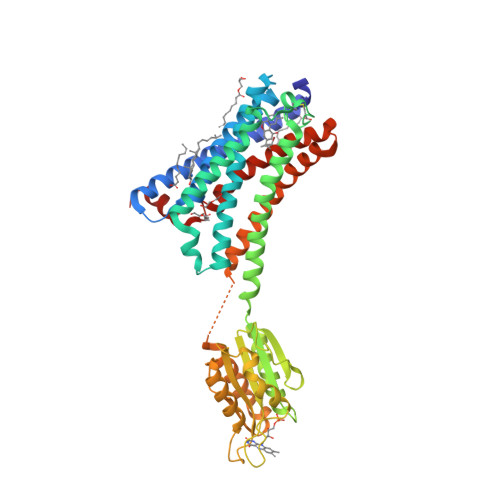
|
|
||||||||||||
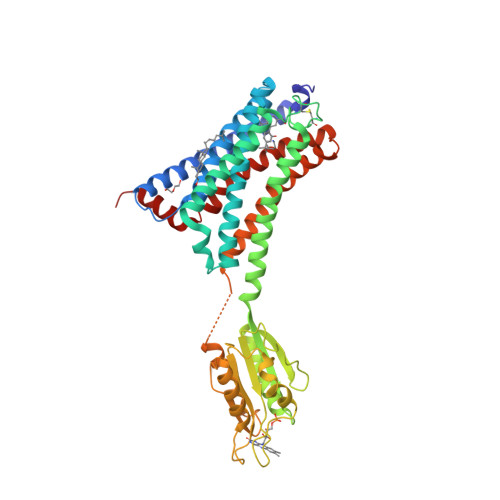
|
|
||||||||||||
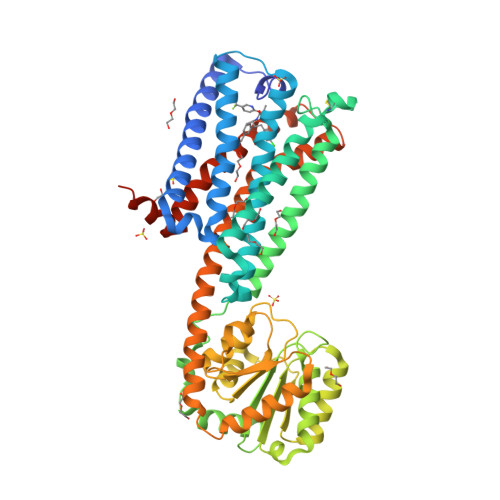
|
|
||||||||||||
|
|
||||||||||||
Natural/Endogenous Ligands  |
| anandamide |
| 2-arachidonoylglycerol |
| Comments: Endogenous ligands include other endocannabinoids |
Download all structure-activity data for this target as a CSV file 
| Agonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific agonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Agonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The higher pKi value of anandamide (7.05) was determined in the presence of phenylmethylsulfonyl fluoride to prevent enzymic hydrolysis. For reviews see references [39,71-72]. The functional activity of a range of synthetic indole cannabinoids is reported by Banister et al. (2015) [1]. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific antagonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AM6538 behaves as a competitive antagonist in functional assays in vitro. It inhibits CP55940- and Δ9-tetrahydrocannabinol (THC)-mediated inhibition of adenylyl cyclase activity and β-arrestin2 recruitment at the human CB1 receptor and [35S]-GTPγS binding in mouse cerebellum [42]. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allosteric Modulators | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific allosteric modulator tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allosteric Modulator Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| It is likely that ZCZ011 can also behave as a positive allosteric modulator of CB1 receptor activation when it is administered in vivo. Thus, evidence was obtained that it can act in vivo to enhance pharmacological effects both of exogenously administered anandamide and CP55940, and of endogenously released anandamide, that are most probably CB1-receptor mediated. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| CB1 receptor is involved in controlling mast cell degranulation and maturation [94], and mediates production of MCP-1 by mast cells which results in recruitment of monocytic myeloid-derived suppressor cells with immunosuppressive outcome [45]. High levels of CB1 expression in the CNS suggest a possible role in neuroinflammation and potential as a drug target [75]. |
| Cell Type Associations | ||||||
|
Primary Transduction Mechanisms 
|
|
| Transducer | Effector/Response |
| Gi/Go family |
Adenylyl cyclase inhibition Potassium channel Calcium channel Other - See Comments |
| Comments: Activation of MAP kinase leading to immediate early gene expression[39] | |
| References: 5,10,24,26,34,40,63-64,70,95,100 | |
Secondary Transduction Mechanisms  |
|
| Transducer | Effector/Response |
| Gs family | Adenylyl cyclase stimulation |
| References: 27,78 | |
Tissue Distribution 
|
||||||||
|
||||||||
|
Expression Datasets  |
|
|
Functional Assays 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Physiological Functions 
|
||||||||
|
||||||||
|
Physiological Consequences of Altering Gene Expression 
|
||||||||||
|
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Biologically Significant Variants 
|
||||||||
|
| General Comments |
| Techniques used include in situ hybridization and immunohistochemistry [74,99]. |
References
1. Banister SD, Stuart J, Kevin RC, Edington A, Longworth M, Wilkinson SM, Beinat C, Buchanan AS, Hibbs DE, Glass M et al.. (2015) Effects of bioisosteric fluorine in synthetic cannabinoid designer drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135. ACS Chem Neurosci, 6 (8): 1445-58. [PMID:25921407]
2. Bauer M, Chicca A, Tamborrini M, Eisen D, Lerner R, Lutz B, Poetz O, Pluschke G, Gertsch J. (2012) Identification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptors. J Biol Chem, 287 (44): 36944-67. [PMID:22952224]
3. Ben-Shabat S, Fride E, Sheskin T, Tamiri T, Rhee MH, Vogel Z, Bisogno T, De Petrocellis L, Di Marzo V, Mechoulam R. (1998) An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol, 353 (1): 23-31. [PMID:9721036]
4. Blaazer AR, Lange JH, van der Neut MA, Mulder A, den Boon FS, Werkman TR, Kruse CG, Wadman WJ. (2011) Novel indole and azaindole (pyrrolopyridine) cannabinoid (CB) receptor agonists: design, synthesis, structure-activity relationships, physicochemical properties and biological activity. Eur J Med Chem, 46 (10): 5086-98. [PMID:21885167]
5. Bouaboula M, Bourrié B, Rinaldi-Carmona M, Shire D, Le Fur G, Casellas P. (1995) Stimulation of cannabinoid receptor CB1 induces krox-24 expression in human astrocytoma cells. J Biol Chem, 270 (23): 13973-80. [PMID:7775459]
6. Bouaboula M, Poinot-Chazel C, Bourrié B, Canat X, Calandra B, Rinaldi-Carmona M, Le Fur G, Casellas P. (1995) Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J, 312 ( Pt 2): 637-41. [PMID:8526880]
7. Bowles NP, Karatsoreos IN, Li X, Vemuri VK, Wood JA, Li Z, Tamashiro KL, Schwartz GJ, Makriyannis AM, Kunos G et al.. (2015) A peripheral endocannabinoid mechanism contributes to glucocorticoid-mediated metabolic syndrome. Proc Natl Acad Sci USA, 112 (1): 285-90. [PMID:25535367]
8. Breivogel CS, Sim LJ, Childers SR. (1997) Regional differences in cannabinoid receptor/G-protein coupling in rat brain. J Pharmacol Exp Ther, 282 (3): 1632-42. [PMID:9316881]
9. Chakrabarti A, Onaivi ES, Chaudhuri G. (1995) Cloning and sequencing of a cDNA encoding the mouse brain-type cannabinoid receptor protein. DNA Seq, 5 (6): 385-8. [PMID:8777318]
10. Childers SR, Deadwyler SA. (1996) Role of cyclic AMP in the actions of cannabinoid receptors. Biochem Pharmacol, 52 (6): 819-27. [PMID:8781498]
11. Chin CN, Lucas-Lenard J, Abadji V, Kendall DA. (1998) Ligand binding and modulation of cyclic AMP levels depend on the chemical nature of residue 192 of the human cannabinoid receptor 1. J Neurochem, 70 (1): 366-73. [PMID:9422383]
12. Chin CN, Murphy JW, Huffman JW, Kendall DA. (1999) The third transmembrane helix of the cannabinoid receptor plays a role in the selectivity of aminoalkylindoles for CB2, peripheral cannabinoid receptor. J Pharmacol Exp Ther, 291 (2): 837-44. [PMID:10525107]
13. Cinar R, Iyer MR, Liu Z, Cao Z, Jourdan T, Erdelyi K, Godlewski G, Szanda G, Liu J, Park JK et al.. (2016) Hybrid inhibitor of peripheral cannabinoid-1 receptors and inducible nitric oxide synthase mitigates liver fibrosis. JCI Insight, 1 (11). [PMID:27525312]
14. Davidson JEP, Dawson CE, Harrison K, Mansell HL, Pratt RM, Sohal S, Ruston VJ. (2004) Azetidinecarboxamide derivatives and their use in the treatment of cb1 receptor mediated disorders. Patent number: WO2004096763A1. Assignee: Vernalis Research Limited. Priority date: 29/04/2004. Publication date: 11/11/2004.
15. De Vry J, Denzer D, Reissmueller E, Eijckenboom M, Heil M, Meier H, Mauler F. (2004) 3-[2-cyano-3-(trifluoromethyl)phenoxy]phenyl-4,4,4-trifluoro-1-butanesulfonate (BAY 59-3074): a novel cannabinoid Cb1/Cb2 receptor partial agonist with antihyperalgesic and antiallodynic effects. J Pharmacol Exp Ther, 310 (2): 620-32. [PMID:15140913]
16. Del Rio C, Cantarero I, Palomares B, Gómez-Cañas M, Fernández-Ruiz J, Pavicic C, García-Martín A, Luz Bellido M, Ortega-Castro R, Pérez-Sánchez C et al.. (2018) VCE-004.3, a cannabidiol aminoquinone derivative, prevents bleomycin-induced skin fibrosis and inflammation through PPARγ- and CB2 receptor-dependent pathways. Br J Pharmacol, 175 (19): 3813-3831. [PMID:30033591]
17. Devane WA, Breuer A, Sheskin T, Järbe TU, Eisen MS, Mechoulam R. (1992) A novel probe for the cannabinoid receptor. J Med Chem, 35 (11): 2065-9. [PMID:1317925]
18. Di Marzo V, Bisogno T, De Petrocellis L, Brandi I, Jefferson RG, Winckler RL, Davis JB, Dasse O, Mahadevan A, Razdan RK, Martin BR. (2001) Highly selective CB1 cannabinoid receptor ligands and novel CB1/VR1 vanilloid receptor "hybrid" ligands. Biochem Biophys Res Commun, 281: 444-451. [PMID:11181068]
19. Dow RL, Carpino PA, Hadcock JR, Black SC, Iredale PA, DaSilva-Jardine P, Schneider SR, Paight ES, Griffith DA, Scott DO et al.. (2009) Discovery of 2-(2-chlorophenyl)-3-(4-chlorophenyl)-7-(2,2-difluoropropyl)-6,7-dihydro-2H-pyrazolo[3,4-f][1,4]oxazepin-8(5H)-one (PF-514273), a novel, bicyclic lactam-based cannabinoid-1 receptor antagonist for the treatment of obesity. J Med Chem, 52 (9): 2652-5. [PMID:19351113]
20. Felder CC, Joyce KE, Briley EM, Glass M, Mackie KP, Fahey KJ, Cullinan GJ, Hunden DC, Johnson DW, Chaney MO et al.. (1998) LY320135, a novel cannabinoid CB1 receptor antagonist, unmasks coupling of the CB1 receptor to stimulation of cAMP accumulation. J Pharmacol Exp Ther, 284 (1): 291-7. [PMID:9435190]
21. Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL. (1995) Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol, 48 (3): 443-50. [PMID:7565624]
22. Felder CC, Veluz JS, Williams HL, Briley EM, Matsuda LA. (1992) Cannabinoid agonists stimulate both receptor- and non-receptor-mediated signal transduction pathways in cells transfected with and expressing cannabinoid receptor clones. Mol Pharmacol, 42 (5): 838-45. [PMID:1331766]
23. Foloppe N, Benwell K, Brooks TD, Kennett G, Knight AR, Misra A, Monck NJ. (2009) Discovery and functional evaluation of diverse novel human CB(1) receptor ligands. Bioorg Med Chem Lett, 19 (15): 4183-90. [PMID:19520572]
24. Galiègue S, Mary S, Marchand J, Dussossoy D, Carrière D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. (1995) Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem, 232 (1): 54-61. [PMID:7556170]
25. Gatley SJ, Lan R, Pyatt B, Gifford AN, Volkow ND, Makriyannis A. (1997) Binding of the non-classical cannabinoid CP 55,940, and the diarylpyrazole AM251 to rodent brain cannabinoid receptors. Life Sci, 61 (14): PL 191-7. [PMID:9335234]
26. Gebremedhin D, Lange AR, Campbell WB, Hillard CJ, Harder DR. (1999) Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am J Physiol, 276 (6): H2085-93. [PMID:10362691]
27. Glass M, Felder CC. (1997) Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci, 17 (14): 5327-33. [PMID:9204917]
28. Griffith DA, Hadcock JR, Black SC, Iredale PA, Carpino PA, DaSilva-Jardine P, Day R, DiBrino J, Dow RL, Landis MS et al.. (2009) Discovery of 1-[9-(4-chlorophenyl)-8-(2-chlorophenyl)-9H-purin-6-yl]-4-ethylaminopiperidine-4-carboxylic acid amide hydrochloride (CP-945,598), a novel, potent, and selective cannabinoid type 1 receptor antagonist. J Med Chem, 52 (2): 234-7. [PMID:19102698]
29. Gérard C, Mollereau C, Vassart G, Parmentier M. (1990) Nucleotide sequence of a human cannabinoid receptor cDNA. Nucleic Acids Res, 18 (23): 7142. [PMID:2263478]
30. Gérard CM, Mollereau C, Vassart G, Parmentier M. (1991) Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J, 279 ( Pt 1): 129-34. [PMID:1718258]
31. Högberg T, Receveur JM, Murray A, Linget JM, Nørregaard PK, Little PB, Cooper M. (2024) Optimizing and characterizing 4-methyl substituted pyrazol-3-carboxamides leading to the peripheral cannabinoid 1 receptor inverse agonist TM38837. Bioorg Med Chem Lett, 98: 129572. [PMID:38043690]
32. Han S, Thatte J, Buzard DJ, Jones RM. (2013) Therapeutic utility of cannabinoid receptor type 2 (CB(2)) selective agonists. J Med Chem, 56 (21): 8224-56. [PMID:23865723]
33. Han S, Thoresen L, Jung JK, Zhu X, Thatte J, Solomon M, Gaidarov I, Unett DJ, Yoon WH, Barden J et al.. (2017) Discovery of APD371: Identification of a Highly Potent and Selective CB2 Agonist for the Treatment of Chronic Pain. ACS Med Chem Lett, 8 (12): 1309-1313. [PMID:29259753]
34. Henry DJ, Chavkin C. (1995) Activation of inwardly rectifying potassium channels (GIRK1) by co-expressed rat brain cannabinoid receptors in Xenopus oocytes. Neurosci Lett, 186 (2-3): 91-4. [PMID:7777206]
35. Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. (1990) Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA, 87 (5): 1932-6. [PMID:2308954]
36. Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, Murphy V, Pertwee RG, Campbell WB. (1999) Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1). J Pharmacol Exp Ther, 289 (3): 1427-33. [PMID:10336536]
37. Hirst RA, Almond SL, Lambert DG. (1996) Characterisation of the rat cerebella CB1 receptor using SR141716A, a central cannabinoid receptor antagonist. Neurosci Lett, 220 (2): 101-4. [PMID:8981483]
38. Hoehe MR, Caenazzo L, Martinez MM, Hsieh WT, Modi WS, Gershon ES, Bonner TI. (1991) Genetic and physical mapping of the human cannabinoid receptor gene to chromosome 6q14-q15. New Biol, 3 (9): 880-5. [PMID:1931832]
39. Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR et al.. (2002) International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev, 54 (2): 161-202. [PMID:12037135]
40. Howlett AC, Qualy JM, Khachatrian LL. (1986) Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol, 29 (3): 307-13. [PMID:2869405]
41. Hua T, Vemuri K, Nikas SP, Laprairie RB, Wu Y, Qu L, Pu M, Korde A, Jiang S, Ho JH et al.. (2017) Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature, 547 (7664): 468-471 RETRACTED. [PMID:28678776]
42. Hua T, Vemuri K, Pu M, Qu L, Han GW, Wu Y, Zhao S, Shui W, Li S, Korde A et al.. (2016) Crystal Structure of the Human Cannabinoid Receptor CB1. Cell, 167 (3): 750-762.e14. [PMID:27768894]
43. Hung MS, Chang CP, Li TC, Yeh TK, Song JS, Lin Y, Wu CH, Kuo PC, Amancha PK, Wong YC et al.. (2010) Discovery of 1-(2,4-dichlorophenyl)-4-ethyl-5-(5-(2-(4-(trifluoromethyl)phenyl)ethynyl)thiophen-2-yl)-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide as a potential peripheral cannabinoid-1 receptor inverse agonist. ChemMedChem, 5 (9): 1439-43. [PMID:20652930]
44. Ignatowska-Jankowska BM, Baillie GL, Kinsey S, Crowe M, Ghosh S, Owens RA, Damaj IM, Poklis J, Wiley JL, Zanda M et al.. (2015) A Cannabinoid CB1 Receptor-Positive Allosteric Modulator Reduces Neuropathic Pain in the Mouse with No Psychoactive Effects. Neuropsychopharmacology, 40 (13): 2948-59. [PMID:26052038]
45. Jackson AR, Hegde VL, Nagarkatti PS, Nagarkatti M. (2014) Characterization of endocannabinoid-mediated induction of myeloid-derived suppressor cells involving mast cells and MCP-1. J Leukoc Biol, 95 (4): 609-19. [PMID:24319288]
46. Jansen EM, Haycock DA, Ward SJ, Seybold VS. (1992) Distribution of cannabinoid receptors in rat brain determined with aminoalkylindoles. Brain Res, 575 (1): 93-102. [PMID:1504787]
47. Jung M, Calassi R, Rinaldi Carmona M, Chardenot P, Le Fur G, Soubrie P, Oury-Donat F. (1997) Characterization of CB1 receptors on rat neuronal cell cultures: binding and functional studies using the selective receptor antagonist SR 141716A. J Neurochem, 68: 402-409. [PMID:8978752]
48. Khanolkar AD, Abadji V, Lin S, Hill WA, Taha G, Abouzid K, Meng Z, Fan P, Makriyannis A. (1996) Head group analogs of arachidonylethanolamide, the endogenous cannabinoid ligand. J Med Chem, 39 (22): 4515-9. [PMID:8893848]
49. Khanolkar AD, Lu D, Ibrahim M, Duclos Jr RI, Thakur GA, Malan Jr TP, Porreca F, Veerappan V, Tian X, George C et al.. (2007) Cannabilactones: a novel class of CB2 selective agonists with peripheral analgesic activity. J Med Chem, 50 (26): 6493-500. [PMID:18038967]
50. Kulkarni PM, Kulkarni AR, Korde A, Tichkule RB, Laprairie RB, Denovan-Wright EM, Zhou H, Janero DR, Zvonok N, Makriyannis A et al.. (2016) Novel Electrophilic and Photoaffinity Covalent Probes for Mapping the Cannabinoid 1 Receptor Allosteric Site(s). J Med Chem, 59 (1): 44-60. [PMID:26529344]
51. Kulkarni S, Nikas SP, Sharma R, Jiang S, Paronis CA, Leonard MZ, Zhang B, Honrao C, Mallipeddi S, Raghav JG et al.. (2016) Novel C-Ring-Hydroxy-Substituted Controlled Deactivation Cannabinergic Analogues. J Med Chem, 59 (14): 6903-19. [PMID:27367336]
52. Lan R, Gatley J, Lu Q, Fan P, Fernando SR, Volkow ND, Pertwee R, Makriyannis A. (1999) Design and synthesis of the CB1 selective cannabinoid antagonist AM281: a potential human SPECT ligand. AAPS PharmSci, 1 (2): E4. [PMID:11741201]
53. Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, Pertwee R, Makriyannis A. (1999) Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem, 42 (4): 769-76. [PMID:10052983]
54. Lange JH, Coolen HK, van Stuivenberg HH, Dijksman JA, Herremans AH, Ronken E, Keizer HG, Tipker K, McCreary AC, Veerman W et al.. (2004) Synthesis, biological properties, and molecular modeling investigations of novel 3,4-diarylpyrazolines as potent and selective CB(1) cannabinoid receptor antagonists. J Med Chem, 47 (3): 627-43. [PMID:14736243]
55. Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM. (2015) Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol, 172 (20): 4790-805. [PMID:26218440]
56. Laprairie RB, Kulkarni AR, Kulkarni PM, Hurst DP, Lynch D, Reggio PH, Janero DR, Pertwee RG, Stevenson LA, Kelly ME et al.. (2016) Mapping Cannabinoid 1 Receptor Allosteric Site(s): Critical Molecular Determinant and Signaling Profile of GAT100, a Novel, Potent, and Irreversibly Binding Probe. ACS Chem Neurosci, 7 (6): 776-98. [PMID:27046127]
57. Laprairie RB, Kulkarni PM, Deschamps JR, Kelly MEM, Janero DR, Cascio MG, Stevenson LA, Pertwee RG, Kenakin TP, Denovan-Wright EM et al.. (2017) Enantiospecific Allosteric Modulation of Cannabinoid 1 Receptor. ACS Chem Neurosci, 8 (6): 1188-1203. [PMID:28103441]
58. Lavey BJ, Kozlowski JA, Hipkin RW, Gonsiorek W, Lundell DJ, Piwinski JJ, Narula S, Lunn CA. (2005) Triaryl bis-sulfones as a new class of cannabinoid CB2 receptor inhibitors: identification of a lead and initial SAR studies. Bioorg Med Chem Lett, 15 (3): 783-6. [PMID:15664857]
59. Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Böhme GA, Imperato A, Pedrazzini T, Roques BP et al.. (1999) Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science, 283 (5400): 401-4. [PMID:9888857]
60. Leleu-Chavain N, Baudelet D, Heloire VM, Rocha DE, Renault N, Barczyk A, Djouina M, Body-Malapel M, Carato P, Millet R. (2019) Benzo[d]thiazol-2(3H)-ones as new potent selective CB2 agonists with anti-inflammatory properties. Eur J Med Chem, 165: 347-362. [PMID:30583970]
61. Liu Z, Iyer MR, Godlewski G, Jourdan T, Liu J, Coffey NJ, Zawatsky CN, Puhl HL, Wess J, Meister J et al.. (2021) Functional Selectivity of a Biased Cannabinoid-1 Receptor (CB1R) Antagonist. ACS Pharmacol Transl Sci, 4 (3): 1175-1187. [PMID:34151207]
62. Lunn CA, Fine JS, Rojas-Triana A, Jackson JV, Fan X, Kung TT, Gonsiorek W, Schwarz MA, Lavey B, Kozlowski JA et al.. (2006) A novel cannabinoid peripheral cannabinoid receptor-selective inverse agonist blocks leukocyte recruitment in vivo. J Pharmacol Exp Ther, 316 (2): 780-8. [PMID:16258021]
63. Mackie K, Hille B. (1992) Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci USA, 89 (9): 3825-9. [PMID:1315042]
64. Mackie K, Lai Y, Westenbroek R, Mitchell R. (1995) Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci, 15: 6552-6561. [PMID:7472417]
65. Mailleux P, Parmentier M, Vanderhaeghen JJ. (1992) Distribution of cannabinoid receptor messenger RNA in the human brain: an in situ hybridization histochemistry with oligonucleotides. Neurosci Lett, 143 (1-2): 200-4. [PMID:1436667]
66. Makriyannis A, Deng H. (2001) Cannabimimetic indole derivatives. Patent number: WO2001028557. Assignee: University Of Connecticut. Priority date: 18/10/1999. Publication date: 26/04/2001.
67. Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ. (1991) Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav, 40 (3): 471-8. [PMID:1666911]
68. Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature, 346 (6284): 561-4. [PMID:2165569]
69. Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR et al.. (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol, 50 (1): 83-90. [PMID:7605349]
70. Pacheco M, Childers SR, Arnold R, Casiano F, Ward SJ. (1991) Aminoalkylindoles: actions on specific G-protein-linked receptors. J Pharmacol Exp Ther, 257 (1): 170-83. [PMID:1902257]
71. Pertwee RG. (1997) Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther, 74 (2): 129-80. [PMID:9336020]
72. Pertwee RG. (1999) Pharmacology of cannabinoid receptor ligands. Curr Med Chem, 6 (8): 635-64. [PMID:10469884]
73. Petitet F, Marin L, Doble A. (1996) Biochemical and pharmacological characterization of cannabinoid binding sites using [3H]SR141716A. Neuroreport, 7: 789-792. [PMID:8733746]
74. Pettit DA, Harrison MP, Olson JM, Spencer RF, Cabral GA. (1998) Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J Neurosci Res, 51 (3): 391-402. [PMID:9486774]
75. Pini A, Mannaioni G, Pellegrini-Giampietro D, Passani MB, Mastroianni R, Bani D, Masini E. (2012) The role of cannabinoids in inflammatory modulation of allergic respiratory disorders, inflammatory pain and ischemic stroke. Curr Drug Targets, 13 (7): 984-93. [PMID:22420307]
76. Piscitelli F, Ligresti A, La Regina G, Coluccia A, Morera L, Allarà M, Novellino E, Di Marzo V, Silvestri R. (2012) Indole-2-carboxamides as allosteric modulators of the cannabinoid CB₁ receptor. J Med Chem, 55 (11): 5627-31. [PMID:22571451]
77. Rangari VA, O'Brien ES, Powers AS, Slivicki RA, Bertels Z, Appourchaux K, Aydin D, Ramos-Gonzalez N, Mwirigi J, Lin L et al.. (2025) A cryptic pocket in CB1 drives peripheral and functional selectivity. Nature, 640 (8057): 265-273. [PMID:40044849]
78. Rhee MH, Bayewitch M, Avidor-Reiss T, Levy R, Vogel Z. (1998) Cannabinoid receptor activation differentially regulates the various adenylyl cyclase isozymes. J Neurochem, 71 (4): 1525-34. [PMID:9751186]
79. Rhee MH, Vogel Z, Barg J, Bayewitch M, Levy R, Hanus L, Breuer A, Mechoulam R. (1997) Cannabinol derivatives: binding to cannabinoid receptors and inhibition of adenylylcyclase. J Med Chem, 40 (20): 3228-33. [PMID:9379442]
80. Rinaldi-Carmona M, Barth F, Congy C, Martinez S, Oustric D, Pério A, Poncelet M, Maruani J, Arnone M, Finance O et al.. (2004) SR147778 [5-(4-bromophenyl)-1-(2,4-dichlorophenyl)-4-ethyl-N-(1-piperidinyl)-1H-pyrazole-3-carboxamide], a new potent and selective antagonist of the CB1 cannabinoid receptor: biochemical and pharmacological characterization. J Pharmacol Exp Ther, 310 (3): 905-14. [PMID:15131245]
81. Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Néliat G, Caput D et al.. (1994) SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett, 350 (2-3): 240-4. [PMID:8070571]
82. Rinaldi-Carmona M, Pialot F, Congy C, Redon E, Barth F, Bachy A, Breliere JC, Soubrie P, Le Fur G. (1996) Characterization and distribution of binding sites for [3H]-SR 141716A, a selective brain (CB1) cannabinoid receptor antagonist, in rodent brain. Life Sci, 58: 1239-1247. [PMID:8614277]
83. Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, Pertwee RG. (1999) Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656 and AM630. Br J Pharmacol, 126: 665-672. [PMID:10188977]
84. Ruiu S, Pinna GA, Marchese G, Mussinu JM, Saba P, Tambaro S, Casti P, Vargiu R, Pani L. (2003) Synthesis and characterization of NESS 0327: a novel putative antagonist of the CB1 cannabinoid receptor. J Pharmacol Exp Ther, 306 (1): 363-70. [PMID:12663689]
85. Schoeder CT, Hess C, Madea B, Meiler J, Müller CE. (2018) Pharmacological evaluation of new constituents of "Spice": synthetic cannabinoids based on indole, indazole, benzimidazole and carbazole scaffolds. Forensic Toxicol, 36 (2): 385-403. [PMID:29963207]
86. Shao Z, Yin J, Chapman K, Grzemska M, Clark L, Wang J, Rosenbaum DM. (2016) High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature, 540 (7634): 602-606. [PMID:27851727]
87. Sharma MK, Murumkar PR, Barmade MA, Giridhar R, Yadav MR. (2015) A comprehensive patents review on cannabinoid 1 receptor antagonists as antiobesity agents. Expert Opin Ther Pat, 25 (10): 1093-116. [PMID:26161824]
88. Shire D, Calandra B, Delpech M, Dumont X, Kaghad M, Le Fur G, Caput D, Ferrara P. (1996) Structural features of the central cannabinoid CB1 receptor involved in the binding of the specific CB1 antagonist SR 141716A. J Biol Chem, 271 (12): 6941-6. [PMID:8636122]
89. Shire D, Calandra B, Rinaldi-Carmona M, Oustric D, Pessègue B, Bonnin-Cabanne O, Le Fur G, Caput D, Ferrara P. (1996) Molecular cloning, expression and function of the murine CB2 peripheral cannabinoid receptor. Biochim Biophys Acta, 1307 (2): 132-6. [PMID:8679694]
90. Shire D, Carillon C, Kaghad M, Calandra B, Rinaldi-Carmona M, Le Fur G, Caput D, Ferrara P. (1995) An amino-terminal variant of the central cannabinoid receptor resulting from alternative splicing. J Biol Chem, 270 (8): 3726-31. [PMID:7876112]
91. Showalter VM, Compton DR, Martin BR, Abood ME. (1996) Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther, 278 (3): 989-99. [PMID:8819477]
92. Sim LJ, Hampson RE, Deadwyler SA, Childers SR. (1996) Effects of chronic treatment with delta9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci, 16 (24): 8057-66. [PMID:8987831]
93. Song ZH, Bonner TI. (1996) A lysine residue of the cannabinoid receptor is critical for receptor recognition by several agonists but not WIN55212-2. Mol Pharmacol, 49 (5): 891-6. [PMID:8622639]
94. Sugawara K, Zákány N, Hundt T, Emelianov V, Tsuruta D, Schäfer C, Kloepper JE, Bíró T, Paus R. (2013) Cannabinoid receptor 1 controls human mucosal-type mast cell degranulation and maturation in situ. J Allergy Clin Immunol, 132 (1): 182-93. [PMID:23453134]
95. Sugiura T, Kodaka T, Kondo S, Tonegawa T, Nakane S, Kishimoto S, Yamashita A, Waku K. (1996) 2-Arachidonoylglycerol, a putative endogenous cannabinoid receptor ligand, induces rapid, transient elevation of intracellular free Ca2+ in neuroblastoma x glioma hybrid NG108-15 cells. Biochem Biophys Res Commun, 229 (1): 58-64. [PMID:8954083]
96. Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T, Szanda G, Mukhopadhyay B, Chedester L, Liow JS et al.. (2012) Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab, 16 (2): 167-79. [PMID:22841573]
97. Thomas BF, Gilliam AF, Burch DF, Roche MJ, Seltzman HH. (1998) Comparative receptor binding analyses of cannabinoid agonists and antagonists. J Pharmacol Exp Ther, 285 (1): 285-92. [PMID:9536023]
98. Thomas BF, Wei X, Martin BR. (1992) Characterization and autoradiographic localization of the cannabinoid binding site in rat brain using [3H]11-OH-delta 9-THC-DMH. J Pharmacol Exp Ther, 263 (3): 1383-90. [PMID:1335065]
99. Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. (1998) Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience, 83: 393-411. [PMID:9460749]
100. Twitchell W, Brown S, Mackie K. (1997) Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol, 78 (1): 43-50. [PMID:9242259]
101. Wiley JL, Martin BR. (2003) Cannabinoid pharmacological properties common to other centrally acting drugs. Eur J Pharmacol, 471 (3): 185-93. [PMID:12826237]
102. Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. (1999) Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA, 96 (10): 5780-5. [PMID:10318961]















