GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Contents:
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | - | 624 | 19p13.2 | KEAP1 | kelch like ECH associated protein 1 | |
| Mouse | - | 624 | 9 A3 | Keap1 | kelch-like ECH-associated protein 1 | |
| Rat | - | 624 | 8q13 | Keap1 | Kelch-like ECH-associated protein 1 | |
Previous and Unofficial Names  |
| INrf2 | kelch-like ECH-associated protein 1 |
Database Links  |
|
| Alphafold | Q14145 (Hs), Q9Z2X8 (Mm), P57790 (Rn) |
| CATH/Gene3D | 2.130.10.80, 2.120.10.80 |
| ChEMBL Target | CHEMBL2069156 (Hs), CHEMBL3562164 (Mm), CHEMBL4523596 (Rn) |
| DrugBank Target | Q14145 (Hs) |
| Ensembl Gene | ENSG00000079999 (Hs), ENSMUSG00000003308 (Mm), ENSRNOG00000020878 (Rn) |
| Entrez Gene | 9817 (Hs), 50868 (Mm), 117519 (Rn) |
| Human Protein Atlas | ENSG00000079999 (Hs) |
| KEGG Gene | hsa:9817 (Hs), mmu:50868 (Mm), rno:117519 (Rn) |
| OMIM | 606016 (Hs) |
| Pharos | Q14145 (Hs) |
| RefSeq Nucleotide | NM_012289 (Hs), NM_016679 (Mm), NM_057152 (Rn) |
| RefSeq Protein | NP_036421 (Hs), NP_057888 (Mm), NP_476493 (Rn) |
| UniProtKB | Q14145 (Hs), Q9Z2X8 (Mm), P57790 (Rn) |
| Wikipedia | KEAP1 (Hs) |
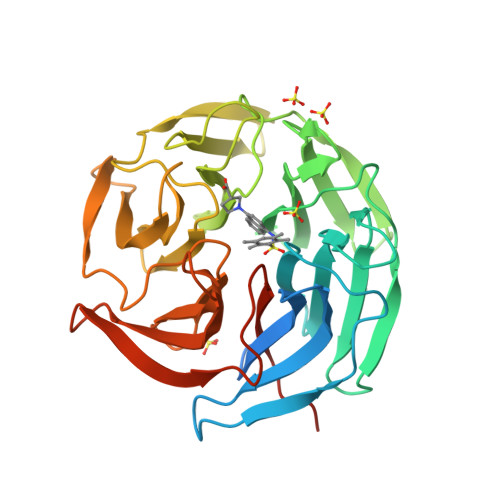
Selected 3D Structures  |
|||||||||||||
|
|
||||||||||||
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other Binding Ligands | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| Transcription of many important detoxification enzymes is regulated by the Nrf2 transcription factor, which is (negatively) regulated by interaction with KEAP1, and when activated by disengagement from KEAP1, enters the nucleus and binds genes with an antioxidant responsive element (ARE) in their promoter sequences. Nrf2-responsive genes play important roles in the cellular defense system, in the regulation of the response to oxidative stress, and facilitate detoxification, antioxidant, cytoprotective and anti-inflammatory functions. Small molecule drug-like compounds that inhibit the KEAP1-Nrf2 protein-protein interaction (a.k.a. Nrf2 activators) are being developed for their potential anti-inflammatory action. The approved MS prodrug dimethyl fumarate was reported to exploit this molecular mechanism, although more recent evidence suggests that its effects may be Nrf2 independent [10]. In order to examine the veracity of Nrf2 activation as an anti-inflammatory modality further, non-electrophilic KEAP1 binding, Nrf2-activating compounds are being developed with the goal of improving on-target selectivity [2-3,9]. |
| General Comments |
| The KEAP1 protein homodimerises and acts as a substrate-recognition adaptor in the formation of the Cullin-RING ligase (CRL) 3 E3 ubiquitin ligase complex. The KEAP1 homodimer substrate is the transcription factor Nrf2 (NF-E2-related factor 2; NFE2L2; Q16236), a master regulator of the antioxidant response. KEAP1 behaves as a repressor that negatively regulates Nrf2 transcriptional activity, by promoting Nrf2 ubiquitination and proteasomal degradation. Direct inhibition of the KEAP1-Nrf2 protein-protein interaction functionally activates transcription of Nrf2 regulated genes and this mechanism is considered a promising strategy for the development of novel drugs with potential antioxidant, anti-inflammatory, and anticancer activities [12]. |
References
1. Cleasby A, Yon J, Day PJ, Richardson C, Tickle IJ, Williams PA, Callahan JF, Carr R, Concha N, Kerns JK et al.. (2014) Structure of the BTB domain of Keap1 and its interaction with the triterpenoid antagonist CDDO. PLoS ONE, 9 (6): e98896. [PMID:24896564]
2. Jain AD, Potteti H, Richardson BG, Kingsley L, Luciano JP, Ryuzoji AF, Lee H, Krunic A, Mesecar AD, Reddy SP et al.. (2015) Probing the structural requirements of non-electrophilic naphthalene-based Nrf2 activators. Eur J Med Chem, 103: 252-68. [PMID:26363505]
3. Jiang ZY, Lu MC, Xu LL, Yang TT, Xi MY, Xu XL, Guo XK, Zhang XJ, You QD, Sun HP. (2014) Discovery of potent Keap1-Nrf2 protein-protein interaction inhibitor based on molecular binding determinants analysis. J Med Chem, 57 (6): 2736-45. [PMID:24512214]
4. Jiang ZY, Xu LL, Lu MC, Chen ZY, Yuan ZW, Xu XL, Guo XK, Zhang XJ, Sun HP, You QD. (2015) Structure-Activity and Structure-Property Relationship and Exploratory in Vivo Evaluation of the Nanomolar Keap1-Nrf2 Protein-Protein Interaction Inhibitor. J Med Chem, 58 (16): 6410-21. [PMID:26258437]
5. Lu M, Ji J, Lv Y, Zhao J, Liu Y, Jiao Q, Liu T, Mou Y, You Q, Jiang Z. (2024) Bivalent inhibitors of the BTB E3 ligase KEAP1 enable instant NRF2 activation to suppress acute inflammatory response. Cell Chem Biol, 31 (6): 1188-1202.e10. [PMID:38157852]
6. Lu MC, Ji JA, Jiang YL, Chen ZY, Yuan ZW, You QD, Jiang ZY. (2016) An inhibitor of the Keap1-Nrf2 protein-protein interaction protects NCM460 colonic cells and alleviates experimental colitis. Sci Rep, 6: 26585. [PMID:27215610]
7. Marcotte D, Zeng W, Hus JC, McKenzie A, Hession C, Jin P, Bergeron C, Lugovskoy A, Enyedy I, Cuervo H et al.. (2013) Small molecules inhibit the interaction of Nrf2 and the Keap1 Kelch domain through a non-covalent mechanism. Bioorg Med Chem, 21 (14): 4011-9. [PMID:23647822]
8. Richardson BG, Jain AD, Potteti HR, Lazzara PR, David BP, Tamatam CR, Choma E, Skowron K, Dye K, Siddiqui Z et al.. (2018) Replacement of a Naphthalene Scaffold in Kelch-like ECH-Associated Protein 1 (KEAP1)/Nuclear Factor (Erythroid-derived 2)-like 2 (NRF2) Inhibitors. J Med Chem, 61 (17): 8029-8047. [PMID:30122040]
9. Richardson BG, Jain AD, Speltz TE, Moore TW. (2015) Non-electrophilic modulators of the canonical Keap1/Nrf2 pathway. Bioorg Med Chem Lett, 25 (11): 2261-8. [PMID:25937010]
10. Schulze-Topphoff U, Varrin-Doyer M, Pekarek K, Spencer CM, Shetty A, Sagan SA, Cree BA, Sobel RA, Wipke BT, Steinman L et al.. (2016) Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc Natl Acad Sci USA, 113 (17): 4777-82. [PMID:27078105]
11. Tran KT, Pallesen JS, Solbak SMØ, Narayanan D, Baig A, Zang J, Aguayo-Orozco A, Carmona RMC, Garcia AD, Bach A. (2019) A Comparative Assessment Study of Known Small-Molecule Keap1-Nrf2 Protein-Protein Interaction Inhibitors: Chemical Synthesis, Binding Properties, and Cellular Activity. J Med Chem, 62 (17): 8028-8052. [PMID:31411465]
12. Wells G. (2015) Peptide and small molecule inhibitors of the Keap1-Nrf2 protein-protein interaction. Biochem Soc Trans, 43 (4): 674-9. [PMID:26551711]
13. Winkel AF, Engel CK, Margerie D, Kannt A, Szillat H, Glombik H, Kallus C, Ruf S, Güssregen S, Riedel J et al.. (2015) Characterization of RA839, a Noncovalent Small Molecule Binder to Keap1 and Selective Activator of Nrf2 Signaling. J Biol Chem, 290 (47): 28446-55. [PMID:26459563]
How to cite this page
Kelch-like proteins: kelch like ECH-associated protein 1. Last modified on 04/01/2024. Accessed on 19/02/2026. IUPHAR/BPS Guide to PHARMACOLOGY, https://www.guidetomalariapharmacology.org/GRAC/ObjectDisplayForward?objectId=2757.