GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Monoamine oxidase A
Target id: 2489
Nomenclature: Monoamine oxidase A
Abbreviated Name: MAO-A
Family: Catecholamine turnover
Contents:
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 1 | 527 | Xp11.3 | MAOA | monoamine oxidase A | |
| Mouse | 1 | 526 | X 11.78 cM | Maoa | monoamine oxidase A | |
| Rat | - | 526 | Xq11 | Maoa | monoamine oxidase A | |
Database Links  |
|
| Alphafold | P21397 (Hs), Q64133 (Mm), G3V9Z3 (Rn) |
| BRENDA | 1.4.3.4 |
| CATH/Gene3D | 3.50.50.60 |
| ChEMBL Target | CHEMBL1951 (Hs), CHEMBL3681 (Mm) |
| DrugBank Target | P21397 (Hs) |
| Ensembl Gene | ENSG00000189221 (Hs), ENSMUSG00000025037 (Mm), ENSRNOG00000002848 (Rn) |
| Entrez Gene | 4128 (Hs), 17161 (Mm), 29253 (Rn) |
| Human Protein Atlas | ENSG00000189221 (Hs) |
| KEGG Enzyme | 1.4.3.4 |
| KEGG Gene | hsa:4128 (Hs), mmu:17161 (Mm), rno:29253 (Rn) |
| Pharos | P21397 (Hs) |
| RefSeq Nucleotide | NM_000240 (Hs), NM_001270458 (Hs), NM_173740 (Mm), NM_033653 (Rn) |
| RefSeq Protein | NP_001257387 (Hs), NP_000231 (Hs), NP_776101 (Mm), NP_387502 (Rn) |
| SynPHARM | 78568 (in complex with clorgiline) |
| UniProtKB | P21397 (Hs), Q64133 (Mm), G3V9Z3 (Rn) |
| Wikipedia | MAOA (Hs) |
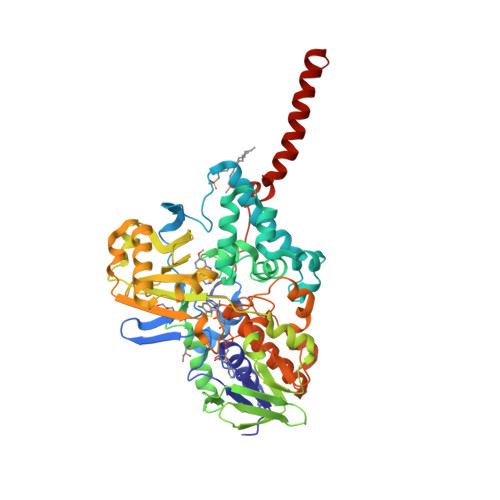
Selected 3D Structures  |
|||||||||||||
|
|
||||||||||||
Enzyme Reaction  |
||||||||||||||||||||||
|
||||||||||||||||||||||
Cofactors  |
||||||||
|
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific inhibitor tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inhibitor Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Differential inhibition of the A or B isozymes of MAO have different clinical outcomes. Inhibition of MAOA results in antidepressant activity [1], whereas inhibition of MAOB results in antiparkinsonian activity [14]. Important substrates for MAO activity in the CNS include dopamine, adrenaline, noradrenaline, serotonin (5-HT), and β-phenylethylamine. Tranylcypromine is an irreversible MAO inhibitor with equal potency for the A and B isozymes [15]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||||
|
||||||||||||
References
1. Binda C, Hubálek F, Li M, Herzig Y, Sterling J, Edmondson DE, Mattevi A. (2004) Crystal structures of monoamine oxidase B in complex with four inhibitors of the N-propargylaminoindan class. J Med Chem, 47 (7): 1767-74. [PMID:15027868]
2. Binda C, Wang J, Li M, Hubalek F, Mattevi A, Edmondson DE. (2008) Structural and mechanistic studies of arylalkylhydrazine inhibition of human monoamine oxidases A and B. Biochemistry, 47 (20): 5616-25. [PMID:18426226]
3. Chaurasiya ND, Gogineni V, Elokely KM, León F, Núñez MJ, Klein ML, Walker LA, Cutler SJ, Tekwani BL. (2016) Isolation of Acacetin from Calea urticifolia with Inhibitory Properties against Human Monoamine Oxidase-A and -B. J Nat Prod, 79 (10): 2538-2544. [PMID:27754693]
4. Curet O, Damoiseau-Ovens G, Sauvage C, Sontag N, Avenet P, Depoortere H, Caille D, Bergis O, Scatton B. (1998) Preclinical profile of befloxatone, a new reversible MAO-A inhibitor. J Affect Disord, 51 (3): 287-303. [PMID:10333983]
5. Ferrari F, Fiorentino S, Mennuni L, Garofalo P, Letari O, Mandelli S, Giordani A, Lanza M, Caselli G. (2011) Analgesic efficacy of CR4056, a novel imidazoline-2 receptor ligand, in rat models of inflammatory and neuropathic pain. J Pain Res, 4: 111-25. [PMID:21647215]
6. Jagrat M, Behera J, Yabanoglu S, Ercan A, Ucar G, Sinha BN, Sankaran V, Basu A, Jayaprakash V. (2011) Pyrazoline based MAO inhibitors: synthesis, biological evaluation and SAR studies. Bioorg Med Chem Lett, 21 (14): 4296-300. [PMID:21680183]
7. Jismy B, El Qami A, Pišlar A, Frlan R, Kos J, Gobec S, Knez D, Abarbri M. (2021) Pyrimido[1,2-b]indazole derivatives: Selective inhibitors of human monoamine oxidase B with neuroprotective activity. Eur J Med Chem, 209: 112911. [PMID:33071056]
8. Lebreton L, Curet O, Gueddari S, Mazouz F, Bernard S, Burstein C, Milcent R. (1995) Selective and potent monoamine oxidase type B inhibitors: 2-substituted 5-aryltetrazole derivatives. J Med Chem, 38 (24): 4786-92. [PMID:7490728]
9. Medvedev AE, Shvedov VI, Chulkova TM, Fedotova OA, Saederup E, Squires RF. (1998) The influence of the antidepressant pirlindole and its dehydro-derivative on the activity of monoamine oxidase A and GABAA receptor binding. J Neural Transm Suppl, 52: 337-42. [PMID:9564636]
10. Mishra N, Sasmal D. (2011) Development of selective and reversible pyrazoline based MAO-B inhibitors: virtual screening, synthesis and biological evaluation. Bioorg Med Chem Lett, 21 (7): 1969-73. [PMID:21377879]
11. Naoi M, Nomura Y, Ishiki R, Suzuki H, Nagatsu T. (1988) 4-(O-benzylphenoxy)-N-methylbutylamine (bifemelane) and other 4-(O-benzylphenoxy)-N-methylalkylamines as new inhibitors of type A and B monoamine oxidase. J Neurochem, 50 (1): 243-7. [PMID:3335842]
12. Phillips OA, D'Silva R, Bahta TO, Sharaf LH, Udo EE, Benov L, Eric Walters D. (2015) Synthesis and biological evaluation of novel 5-(hydroxamic acid)methyl oxazolidinone derivatives. Eur J Med Chem, 106: 120-31. [PMID:26536532]
13. Son SY, Ma J, Kondou Y, Yoshimura M, Yamashita E, Tsukihara T. (2008) Structure of human monoamine oxidase A at 2.2-A resolution: the control of opening the entry for substrates/inhibitors. Proc Natl Acad Sci USA, 105 (15): 5739-44. [PMID:18391214]
14. Tetrud JW, Langston JW. (1989) The effect of deprenyl (selegiline) on the natural history of Parkinson's disease. Science, 245 (4917): 519-22. [PMID:2502843]
15. Yoshida S, Rosen TC, Meyer OG, Sloan MJ, Ye S, Haufe G, Kirk KL. (2004) Fluorinated phenylcyclopropylamines. Part 3: Inhibition of monoamine oxidase A and B. Bioorg Med Chem, 12 (10): 2645-52. [PMID:15110846]
How to cite this page
Catecholamine turnover: Monoamine oxidase A. Last modified on 27/02/2025. Accessed on 07/03/2026. IUPHAR/BPS Guide to PHARMACOLOGY, https://www.guidetomalariapharmacology.org/GRAC/ObjectDisplayForward?objectId=2489.