GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
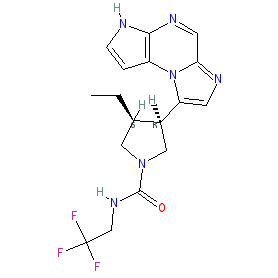
Synonyms: ABT-494 | Rinvoq®
upadacitinib is an approved drug (EMA & FDA (2019))
Compound class:
Synthetic organic
Comment: Upadacitinib (ABT-494) is a novel second generation orally active Janus kinase inhibitor with high JAK1 selectivity [5,10]. The chemical structure is claimed as compound 1 in Abbvie's patent WO2015061665 [10]. It was developed for potential clinical efficacy in multiple autoimmune diseases. Its first approval was for the treatment of rheumatoid arthritis [2].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Abbvie press release.
Upadacitinib Meets All Primary and Ranked Secondary Endpoints Including Superiority Versus Adalimumab in Phase 3 Study in Rheumatoid Arthritis. Accessed on 12/04/2018. Modified on 12/04/2018. https://news.abbvie.com, https://news.abbvie.com/news/upadacitinib-meets-all-primary-and-ranked-secondary-endpoints-including-superiority-versus-adalimumab-in-phase-3-study-in-rheumatoid-arthritis.htm |
|
2. Duggan S, Keam SJ. (2019)
Upadacitinib: First Approval. Drugs, 79 (16): 1819-1828. [PMID:31642025] |
|
3. Fleischmann R, Pangan AL, Song IH, Mysler E, Bessette L, Peterfy C, Durez P, Ostor AJ, Li Y, Zhou Y et al.. (2019)
Upadacitinib Versus Placebo or Adalimumab in Patients With Rheumatoid Arthritis and an Inadequate Response to Methotrexate: Results of a Phase III, Double-Blind, Randomized Controlled Trial. Arthritis Rheumatol, 71 (11): 1788-1800. [PMID:31287230] |
|
4. Mohamed MF, Klünder B, Camp HS, Othman AA. (2019)
Exposure-Response Analyses of Upadacitinib Efficacy in Phase II Trials in Rheumatoid Arthritis and Basis for Phase III Dose Selection. Clin Pharmacol Ther, 106 (6): 1319-1327. [PMID:31194885] |
|
5. Parmentier JM, Voss J, Graff C, Schwartz A, Argiriadi M, Friedman M, Camp HS, Padley RJ, George JS, Hyland D et al.. (2018)
In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol, 2: 23. [PMID:30886973] |
|
6. Pérez-Jeldres T, Tyler CJ, Boyer JD, Karuppuchamy T, Yarur A, Giles DA, Yeasmin S, Lundborg L, Sandborn WJ, Patel DR et al.. (2019)
Targeting Cytokine Signaling and Lymphocyte Traffic via Small Molecules in Inflammatory Bowel Disease: JAK Inhibitors and S1PR Agonists. Front Pharmacol, 10: 212. [PMID:30930775] |
|
7. Shukla T, Sands BE. (2019)
Novel Non-biologic Targets for Inflammatory Bowel Disease. Curr Gastroenterol Rep, 21 (5): 22. [PMID:31016396] |
|
8. Smolen JS, Pangan AL, Emery P, Rigby W, Tanaka Y, Vargas JI, Zhang Y, Damjanov N, Friedman A, Othman AA et al.. (2019)
Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet, 393 (10188): 2303-2311. [PMID:31130260] |
|
9. Torgutalp M, Poddubnyy D. (2018)
Emerging treatment options for spondyloarthritis. Best Pract Res Clin Rheumatol, 32 (3): 472-484. [PMID:31171316] |
|
10. Voss JW, Camp HS, Padley RJ. (2015)
Jak1 selective inhibitor and uses thereof. Patent number: WO2015061665. Assignee: Abbvie Inc.. Priority date: 24/10/2013. Publication date: 30/04/2015. |
|
11.
AbbVie's Upadacitinib (ABT-494) Meets All Primary and Ranked Secondary Endpoints in Second Phase 3 Study in Rheumatoid Arthritis.
Accessed on 18/09/2017. Modified on 18/09/2017. drugs.com, https://www.drugs.com/clinical_trials/abbvie-s-upadacitinib-abt-494-meets-all-primary-ranked-secondary-endpoints-second-phase-3-study-17607.html |