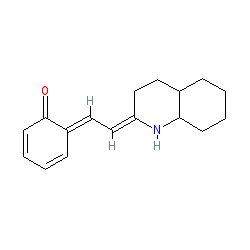
|
Synonyms: Cpd 3 (PMID 24990314)
Compound class:
Synthetic organic
Comment: ECE-2 inhibitor. Unlike ECE-1, this protease has a restricted neuroendocrine distribution, an acidic pH optimum and may regulate neuropeptide levels [1]. Note there are a number of tautomeric forms in PubChem that vary in the double bond renderings. Note the publication that introduces the name S136492 [2] does not make the structure explicit but refers to the earlier paper [1].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Gagnidze K, Sachchidanand, Rozenfeld R, Mezei M, Zhou MM, Devi LA. (2008)
Homology modeling and site-directed mutagenesis to identify selective inhibitors of endothelin-converting enzyme-2. J Med Chem, 51 (12): 3378-87. [PMID:18507370] |
|
2. Gupta A, Fujita W, Gomes I, Bobeck E, Devi LA. (2015)
Endothelin-converting enzyme 2 differentially regulates opioid receptor activity. Br J Pharmacol, 172 (2): 704-19. [PMID:24990314] |