GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
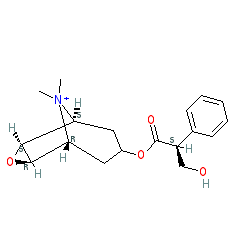
Abbreviated name: NMS
Synonyms: methylscopolamine | methylscopolamine bromide | N-methylscopolamine | Pamine®
N-methyl scopolamine is an approved drug (FDA (1953))
Compound class:
Synthetic organic
Comment: The approved drug methylscopolamine bromide consists of N-methyl scopolamine and bromide ions. There is some ambiguity in the literature and in other databases surrounding the exact stereochemistry of N-methyl scopolamine. Alternative representations include CHEMBL3140030 and CHEMBL376897.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Dowling MR, Charlton SJ. (2006)
Quantifying the association and dissociation rates of unlabelled antagonists at the muscarinic M3 receptor. Br J Pharmacol, 148 (7): 927-37. [PMID:16847442] |
|
2. Fruchart-Gaillard C, Mourier G, Marquer C, Ménez A, Servent D. (2006)
Identification of various allosteric interaction sites on M1 muscarinic receptor using 125I-Met35-oxidized muscarinic toxin 7. Mol Pharmacol, 69 (5): 1641-51. [PMID:16439611] |