GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
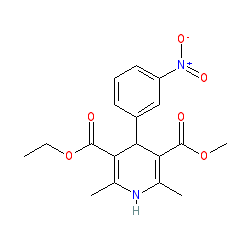
nitrendipine is an approved drug
Compound class:
Synthetic organic
Comment: The approved drug nitrendipine is a racemic mixture of two enantiomers; (R)-nitrendipine and (S)-nitrendipine. The structure shown here does not specify stereochemistry and represents the mixture.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Beam KG, Knudson CM. (1988)
Calcium currents in embryonic and neonatal mammalian skeletal muscle. J Gen Physiol, 91 (6): 781-98. [PMID:2458429] |
|
2. Bean BP. (1984)
Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci USA, 81 (20): 6388-92. [PMID:6093100] |
|
3. Jensen BS, Strobaek D, Christophersen P, Jorgensen TD, Hansen C, Silahtaroglu A, Olesen SP, Ahring PK. (1998)
Characterization of the cloned human intermediate-conductance Ca2+-activated K+ channel. Am J Physiol, 275 (3): C848-56. [PMID:9730970] |
|
4. Sinnegger-Brauns MJ, Huber IG, Koschak A, Wild C, Obermair GJ, Einzinger U, Hoda JC, Sartori SB, Striessnig J. (2009)
Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol Pharmacol, 75 (2): 407-14. [PMID:19029287] |
|
5. Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. (2000)
Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci USA, 97 (14): 8151-6. [PMID:10884437] |