|
Compound class:
Synthetic organic
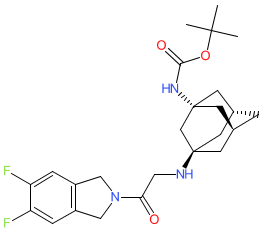
Comment: This ligand, derived from vildagliptin, has been optimised to exhibit selectivity for dipeptidyl peptidase 9 (DPP9) compared to the structurarly similar enzyme DPP8 [1]. It is intended as a tool to further the investigation of the role of DPP9 in cellular processes such as NLRP1- and CARD8-mediated inflammatory cell death (pyroptosis), and to help determine specific physiological and pathological functions for each enzyme.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Benramdane S, De Loose J, Filippi N, Espadinha M, Beyens O, Rymenant YV, Dirkx L, Bozdag M, Feijens PB, Augustyns K et al.. (2023)
Highly Selective Inhibitors of Dipeptidyl Peptidase 9 (DPP9) Derived from the Clinically Used DPP4-Inhibitor Vildagliptin. J Med Chem, 66 (18): 12717-12738. [PMID:37721854] |