|
Synonyms: 6059-S | LY127935 | moxalactam | Moxam®
latamoxef is an approved drug (FDA (1981))
Compound class:
Synthetic organic
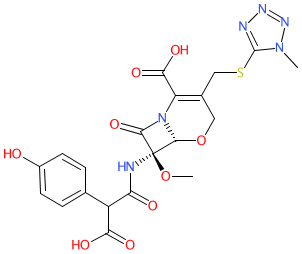
Comment: Latamoxef is an oxacephem molecule developed by the Shionogi Research Laboratories (Osaka, Japan) [3]. It belongs to the β-lactam class of antibacterial compounds.
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Brown RB, Klar J, Lemeshow S, Teres D, Pastides H, Sands M. (1986)
Enhanced bleeding with cefoxitin or moxalactam. Statistical analysis within a defined population of 1493 patients. Arch Intern Med, 146 (11): 2159-64. [PMID:3778044] |
|
2. Carmine AA, Brogden RN, Heel RC, Romankiewicz JA, Speight TM, Avery GS. (1983)
Moxalactam (latamoxef). A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs, 26 (4): 279-333. [PMID:6354685] |
|
3. Otsuka H, Nagata W, Yoshioka M, Narisada M, Yoshida T, Harada Y, Yamada H. (1981)
Discovery and development of Moxalactam (6059-S): the chemistry and biology of 1-oxacephems. Med Res Rev, 1 (3): 217-48. [PMID:6213825] |
|
4. Weitekamp MR, Aber RC. (1983)
Prolonged bleeding times and bleeding diathesis associated with moxalactam administration. JAMA, 249 (1): 69-71. [PMID:6217353] |