GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
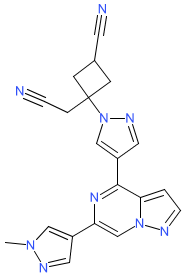
Synonyms: compound 22 [PMID: 32787094] | PF-06826647 | PF06826647 | Tyk2-IN-8
Compound class:
Synthetic organic
Comment: Ropsacitinib (PF-06826647) is claimed as a tyrosine kinase 2 (TYK2) Inhibitor [1]. However, its activity at clinically relevant concentrations to achieve TYK2 inhibition is suggestive of significant inhibition of at least JAK2, if not also of JAK1 [3]. Ropsacitinib is an ATP-competitive inhibitor.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Gerstenberger BS, Ambler C, Arnold EP, Banker ME, Brown MF, Clark JD, Dermenci A, Dowty ME, Fensome A, Fish S et al.. (2020)
Discovery of Tyrosine Kinase 2 (TYK2) Inhibitor (PF-06826647) for the Treatment of Autoimmune Diseases. J Med Chem, 63 (22): 13561-13577. [PMID:32787094] |
|
2. Singh RSP, Pradhan V, Roberts ES, Scaramozza M, Kieras E, Gale JD, Peeva E, Vincent MS, Banerjee A, Fensome A et al.. (2021)
Safety and Pharmacokinetics of the Oral TYK2 Inhibitor PF-06826647: A Phase I, Randomized, Double-Blind, Placebo-Controlled, Dose-Escalation Study. Clin Transl Sci, 14 (2): 671-682. [PMID:33290616] |
|
3. Tehlirian C, Peeva E, Kieras E, Scaramozza M, Roberts ES, Singh RSP, Pradhan V, Banerjee A, Garcet S, Xi L et al.. (2021)
Safety, tolerability, efficacy, pharmacokinetics, and pharmacodynamics of the oral TYK2 inhibitor PF-06826647 in participants with plaque psoriasis: a phase 1, randomised, double-blind, placebo-controlled, parallel-group study. Lancet Rheumatology, 3 (3): e204-e21. DOI: 10.1016/S2665-9913(20)30397-0 |