GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
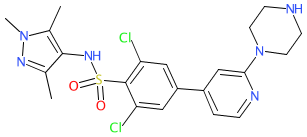
Synonyms: compound 1a [PMID: 24451586] | IMP-366
Compound class:
Synthetic organic
Comment: The pyrazole sulfonamide, DDD85646, was first identified as a potent N-myristoyltransferase (NMT) inhibitor in T. brucei, the parasite that causes African sleeping sickness (trypanosomiasis) [1]. The compound also has antimalarial activity [3].
The Malaria tab on this ligand page provides additional curator comments of relevance to the Guide to MALARIA PHARMACOLOGY. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Frearson JA, Brand S, McElroy SP, Cleghorn LA, Smid O, Stojanovski L, Price HP, Guther ML, Torrie LS, Robinson DA et al.. (2010)
N-myristoyltransferase inhibitors as new leads to treat sleeping sickness. Nature, 464 (7289): 728-32. [PMID:20360736] |
|
2. Schlott AC, Mayclin S, Reers AR, Coburn-Flynn O, Bell AS, Green J, Knuepfer E, Charter D, Bonnert R, Campo B et al.. (2019)
Structure-Guided Identification of Resistance Breaking Antimalarial N‑Myristoyltransferase Inhibitors. Cell Chem Biol, 26 (7): 991-1000.e7. [PMID:31080074] |
|
3. Wright MH, Clough B, Rackham MD, Rangachari K, Brannigan JA, Grainger M, Moss DK, Bottrill AR, Heal WP, Broncel M et al.. (2014)
Validation of N-myristoyltransferase as an antimalarial drug target using an integrated chemical biology approach. Nat Chem, 6 (2): 112-21. [PMID:24451586] |