GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: SAR97276 | SAR97276A | T3
Compound class:
Synthetic organic
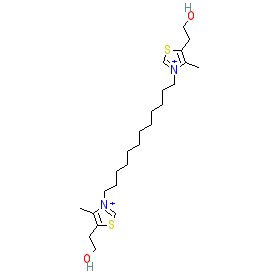
Comment: Albitiazolium was the clinical lead from the bisthiazolium series, a novel class of choline analogue with potent antimalarial activity [2-3].
We display the structure of the parent compound, with PubChem and ChEMBL links provided in the table below. However, the INN-assigned compound is in complex with bromide (represented in PubChem CID 11377022) and this compound is used in the experimental data listed under the 'biological activity' tab. The Malaria tab on this ligand page provides additional curator comments of relevance to the Guide to MALARIA PHARMACOLOGY. |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Held J, Supan C, Salazar CLO, Tinto H, Bonkian LN, Nahum A, Sié A, Abdulla S, Cantalloube C, Djeriou E et al.. (2017)
Safety and efficacy of the choline analogue SAR97276 for malaria treatment: results of two phase 2, open-label, multicenter trials in African patients. Malar J, 16 (1): 188. [PMID:28472957] |
|
2. Nicolas O, Margout D, Taudon N, Wein S, Calas M, Vial HJ, Bressolle FM. (2005)
Pharmacological properties of a new antimalarial bisthiazolium salt, T3, and a corresponding prodrug, TE3. Antimicrob Agents Chemother, 49 (9): 3631-9. [PMID:16127032] |
|
3. Vial HJ, Wein S, Farenc C, Kocken C, Nicolas O, Ancelin ML, Bressolle F, Thomas A, Calas M. (2004)
Prodrugs of bisthiazolium salts are orally potent antimalarials. Proc Natl Acad Sci USA, 101 (43): 15458-63. [PMID:15492221] |
|
4. Wein S, Maynadier M, Bordat Y, Perez J, Maheshwari S, Bette-Bobillo P, Tran Van Ba C, Penarete-Vargas D, Fraisse L, Cerdan R et al.. (2012)
Transport and pharmacodynamics of albitiazolium, an antimalarial drug candidate. Br J Pharmacol, 166 (8): 2263-76. [PMID:22471905] |