GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
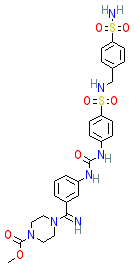
Compound class:
Synthetic organic
Comment: SC83288 is an amicarbalide derivative and the clinical development candidate for this novel antimalarial chemotype [2].
The Malaria tab on this ligand page provides additional curator comments of relevance to the Guide to MALARIA PHARMACOLOGY. |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Duffey M, Sanchez CP, Lanzer M. (2018)
Profiling of the anti-malarial drug candidate SC83288 against artemisinins in Plasmodium falciparum. Malar J, 17 (1): 121. [PMID:29558913] |
|
2. Pegoraro S, Duffey M, Otto TD, Wang Y, Rösemann R, Baumgartner R, Fehler SK, Lucantoni L, Avery VM, Moreno-Sabater A et al.. (2017)
SC83288 is a clinical development candidate for the treatment of severe malaria. Nat Commun, 8: 14193. [PMID:28139658] |