GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
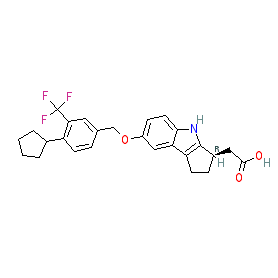
Synonyms: APD-334 | APD334 | APD334-003 | compound 4 [PMID: 25516790] | Velsipity®
etrasimod is an approved drug (FDA (2023), EMA (2024))
Compound class:
Synthetic organic
Comment: Etrasimod (APD334) is a centrally available, selective S1P1 receptor agonist [2]. APD334 is being investigated for clinical efficacy as an immunosuppressant for the treatment of autoimmune diseases.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Immunopharmacology Disease | |||
| Disease | X-Refs | Comment | References |
| Pyoderma gangrenosum |
Disease Ontology:
DOID:8553 Orphanet: ORPHA48104 |
Phase 2 clinical candidate for this condition (see NCT03072953). | |
| Inflammatory bowel disease |
Disease Ontology:
DOID:0050589 OMIM: 266600 |
Phase 2 clinical candidate for IBD patients (including UC and Crohn's disease) with active skin manifestations (see proof of concept study NCT03139032). | |