|
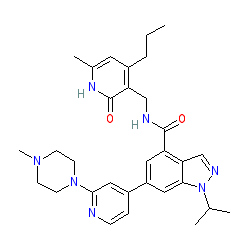
Synonyms: compound 6 [PMID 24900432] [2] | GSK 343
Compound class:
Synthetic organic
Comment: GSK343 is a cell-permeable inhibitor of EZH2 histone H3 methyltransferase activity, developed by GlaxoSmithKline and included in the Structural Genomics Consortium's (SGCs) portfilio of epigenetic probe compounds [1-2].
Click here to link to the SGC's full list of epigenetics probes. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| GSK343 has been used to demonstrate that inhibition of EZH2 alleviates experimental intestinal inflammation [3]. In this study pharmacological inhibition of EZH2 increased the population of functional myeloid-derived suppressor cells (MDSCs) in the gut, which in turn produced immunosuppressive disease-modifying effects. These findings identify EZH2 inhibition as a potential therapeutic approach for the treatment of inflammatory bowel disease. The authors also caution that the use of EZH2 inhibitors in oncology may suppress beneficial anticancer immunity (by increasing MDSC populations). |