|
Synonyms: RG-7625 | RG7625 | RO-5459072
Compound class:
Synthetic organic
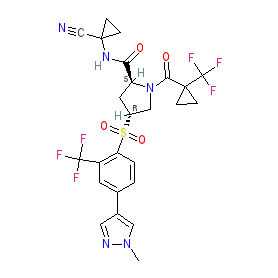
Comment: Petesicatib is the INN for a cathepsin S inhibitor. The structure (and CAS registry number) submitted to the WHO match PubChem CID 59543597, and this ties the INN together with the research code RO5459072 assigned by Hoffman-La Roche to their cathepsin S inhibitor [2-3]. This same chemical structure is claimed in Hoffmann-La Roche patent WO2010121918A1, where it is example 238 [1].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Petesicatib (RO5459072 or RG7625) has completed Phase 2 clinical evaluation in Sjögren's syndrome (NCT02701985) and Phase 1 in celiac disease (NCT02679014), but there are no active clinical trials registered with ClinicalTrials.gov. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02679014 | A Study to Investigate the Pharmacokinetics, Pharmacodynamic Effects, Safety and Tolerability of Repeated Dosing of RO5459072 in Volunteers With Celiac Disease | Phase 1 Interventional | Hoffmann-La Roche | ||
| NCT02701985 | A Study to Assess the Efficacy of RO5459072 in Participants With Primary Sjogren's Syndrome | Phase 2 Interventional | Hoffmann-La Roche | ||