GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
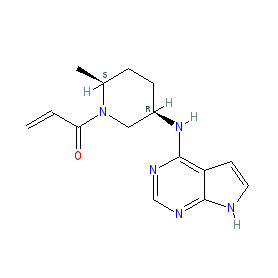
Synonyms: compound 11 [PMID: 28139931] | example 5 [WO2015083028] | Litfulo® | PF-06651600 | PF06651600
ritlecitinib is an approved drug (FDA, UK & EMA (2023))
Compound class:
Synthetic organic
Comment: Ritlecitinib (PF-06651600) is a potent, orally active, molecule with dual inhibitor activities. It acts as a covalent and selective inhibitor of Janus kinase 3 (JAK3) [7] (a type I inhibitor, that binds to the kinase in its ATP pocket), and it also inhibits TEC family kinases (BTK, ITK, TEC, Etk, TXK) that are involved in immune cell regulation. It has demonstrated anti-inflammatory activities in in vivo models [6]. Ritlecitinib is example 5 in a Pfizer patent that provides SAR for 343 analogues [1]. There are three crystal structures available for compounds reported in [7] with JAK3, but not for compound 11 (the PDB identifiers are 5TTV, 5TTU and 5TTS).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| PF-06651600 completed Phase 2 clinical trials for rheumatoid arthritis and ulcerative colitis and was advanced to Phase 2b/3 in alopecia areata patients (the ALLEGRO study). Click here to link to ClinicalTrials.gov's full list of PF-06651600 trials. In June 2023 the FDA approved ritlecitinib to treat severe alopecia areata, based on positive efficacy results from the ALLEGRO study [3]. The UK's HMRA approved the drug for this indication in November 2023. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02958865 | Study to Compare Oral PF-06651600, PF-06700841 and Placebo in Subjects With Moderate to Severe Ulcerative Colitis | Phase 2 Interventional | Pfizer | 5 | |
| NCT03732807 | PF-06651600 for the Treatment of Alopecia Areata | Phase 2/Phase 3 Interventional | Pfizer | Results from this study suggest that ritlecitinib might be a suitable systermic treatment option for alopecia areata. It was effective and well tolerated, with similar rates of adverse events in the ritlecitinib and placebo groups. | 3 |
External links  |
|
For extended ADME data see the following: European Medicines Agency (EMA) |