GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
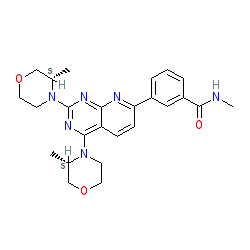
Synonyms: AZD 2014 | AZD-2014 | AZD2014 | cc-551
Compound class:
Synthetic organic
Comment: Vistusertib (AZD2014) is a selective inhibitor of Mammalian target of rapamycin (mTOR) serine/ threonine kinase. This compound is included in AstaZeneca's Open Innovation Pharmacology Toolbox.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Vistusertib (AZD2014) is being evaluated in more than 10 Phase 2 clinical trials, for various cancers. Indications include advanced/metastatic breast cancers, glioblastoma multiforme, metastatic clear cell renal carcinoma, (metastatic) squamous non-small cell lung cancer, advanced ovarian cancer and advanced gastric adenocarcinoma. Current investigations favour a combination based approach, for example vistusertib and durvalumab in bladder cancer (Phase 1 NCT02546661) and vistusertib plus rituximab in relapsed/refractory diffuse large B cell lymphoma (Phase 2 NCT02752204). Having shown promise in preclinical animal models of ovarian cancer [8] a combination of vistusertib plus paclitaxel was advanced to human trial in patients with high grade serous ovarian and squamous non-small cell lung cancers. Phase 1 results from this trial (NCT02193633) were published by Basu et al. (2018) [1]. In both cancer types the dual therapy regimen achieved a median progression-free survival of 5.8 months (note: the daily vistusertib dose had to be reduced by half, to 25mg, to manage toxicities in the lung cancer patients). Click here to link to ClinicalTrials.gov's full list of AZD2014 trials. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02193633 | A Phase I Trial of the Combination of AZD2014 and Weekly Paclitaxel. | Phase 1 Interventional | Royal Marsden NHS Foundation Trust | ||
| NCT02546661 | Open-Label, Randomised, Multi-Drug, Biomarker-Directed, Phase 1b Study in Pts w/ Muscle Invasive Bladder Cancer | Phase 1 Interventional | AstraZeneca | ||
| NCT02752204 | An Evaluation AZD2014 Alone and in Combination With Rituximab in Relapsed/Refractory Diffuse Large B Cell Lymphoma | Phase 2 Interventional | University of Birmingham | ||