|
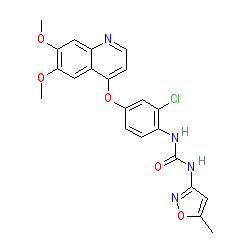
Synonyms: AV-951 | AV951 | Fotivda® | KRN-951 | KRN951 | VEGFR tyrosine kinase inhibitor IV
tivozanib is an approved drug (EMA (2017), FDA (2021))
Compound class:
Synthetic organic
Comment: Tivozanib is an oral VEGF receptor tyrosine kinase inhibitor.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Tivozanib was approved by the EMA in 2017, and it is also approved for use in Norway and Iceland. Tivozanib is indicated as a first line treatment of adult patients with advanced renal cell carcinoma (RCC) and for adult patients who are VEGFR and mTOR pathway inhibitor-naïve following disease progression after one prior treatment with cytokine therapy for advanced RCC. Click here to view all tivozanib studies that are currently listed on ClinicalTrials.gov. The first FDA approval for tivozanib was granted in March 2021; indicated for advanced RCC that has relapsed or is refractory following ≥2 systemic prior therapies. |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) European Medicines Agency (EMA) |