GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
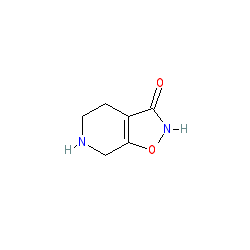
Synonyms: LU-02030 | MK-0928 | MK0928 | OV101 | THIP
Compound class:
Synthetic organic
Comment: GABAA agonist, with some preference for δ subunit-containing channels [6].
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Gaboxadol was investigated as a treatment for insomnia. More recently it has been repositioned for Fragile X and Angelman syndromes based on evidence that targeting GABAAergic system deficit can improve behavioural abnormalities in these syndromes [2,4-5,10]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT00102167 | Study of MK0928 in Healthy Adult Volunteers in a Model of Insomnia (0928-006)(COMPLETED) | Phase 3 Interventional | H. Lundbeck A/S | ||
| NCT03697161 | A Study of OV101 in Individuals With Fragile X Syndrome | Phase 2 Interventional | Ovid Therapeutics Inc. | ||
| NCT06334419 | Placebo-Controlled, Single-Dose Challenge Study of Gaboxadol in Adult Males With Fragile X Syndrome (FXS) | Phase 2 Interventional | Children's Hospital Medical Center, Cincinnati | ||
| NCT02996305 | A Study in Adults and Adolescents With Angelman Syndrome (STARS) | Phase 2 Interventional | Ovid Therapeutics Inc. | 1 | |
| NCT04106557 | A Study of OV101 in Individuals With Angelman Syndrome (AS) | Phase 3 Interventional | Ovid Therapeutics Inc. | This study in pediatric participants with angelman syndrome found no improvement in symptom severity scores between placebo and gaboxadol after 12 weeks of treament. | 7 |