|
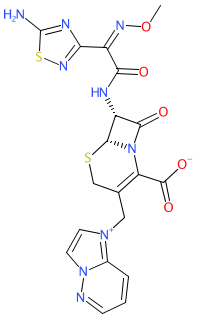
Synonyms: SCE-2787
cefozopran is an approved drug (Japan)
Compound class:
Synthetic organic
Comment: Cefozopran is a fourth generation cephalosporin, belonging to the β-lactam class of antibacterial compounds. It has broad-spectrum activity and was developed by Takeda for parenteral treatment of infections caused by a range of Gram-positive and Gram-negative bacteria [1-2].
|
|
|||||||||||||||||||||||||||||||||||