|
Compound class:
Synthetic organic
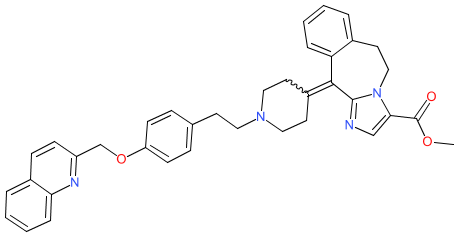
Comment: Laniquidar (R101933) is a third generation inhibitor of the ATP-binding cassette transporter P-glycoprotein (P-gp; ABCB1) [1-2]. P-gp is a drug transporter and efflux pump that can promote resistance to drugs, including chemotherapeutics. Pharmacologic inhibition of P-gp is an established strategy that is being investigated to block this resistance mechanism in cancers [1].
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT00028873 | R101933 Combined With Chemotherapy in Treating Patients With Metastatic Breast Cancer That Has Not Responded to Previous Chemotherapy | Phase 2 Interventional | European Organisation for Research and Treatment of Cancer - EORTC | ||